Role and regulation of growth plate vascularization during coupling with osteogenesis in tibial dyschondroplasia of chickens
- PMID: 29487404
- PMCID: PMC5829164
- DOI: 10.1038/s41598-018-22109-y
Role and regulation of growth plate vascularization during coupling with osteogenesis in tibial dyschondroplasia of chickens
Abstract
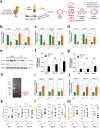
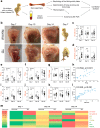
Tibial dyschondroplasia (TD) is the most-prevalent leg disorder in fast-growing chickens; it is intractable and characterized by abnormal endochondral bone formation of proximal tibial growth-plates (TGPs). Previous studies have shown that bone is a highly vascularized tissue dependent on the coordinated coupling between angiogenesis and osteogenesis, but the underlying mechanisms of bone formation and bone remodeling are poorly defined in TD chickens. Here, we observed that inhibition of vasculogenesis and angiogenesis remarkably impaired vascular invasion in the hypertrophic chondrocyte zone of the TGPs, resulting in the massive death of chondrocytes due to a shortage of blood vessels and nutrients. Moreover, the balance of the OPG (osteoprotegerin)/RANKL (receptor activator of nuclear factor-kB ligand) system is also severely disrupted during the osteogenesis process while coupling with angiogenesis, both of which eventually lead to abnormal endochondral bone formation in TD chickens. Thus, the process of vascular formation in endochondral bone appears to initiate the pathological changes in TD, and improvement of this process during coupling with osteogenesis may be a potential therapeutic approach to treat this intractable disease.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Hypoxia, hypoxia-inducible factor-1alpha (HIF-1alpha), and heat-shock proteins in tibial dyschondroplasia.Poult Sci. 2008 Aug;87(8):1556-64. doi: 10.3382/ps.2008-00124. Poult Sci. 2008. PMID: 18648049
-
Changes in the tibial growth plates of chickens with thiram-induced dyschondroplasia.J Comp Pathol. 2005 Jul;133(1):41-52. doi: 10.1016/j.jcpa.2005.01.005. J Comp Pathol. 2005. PMID: 15899490
-
The role of blood vessels in broiler chickens with tibial dyschondroplasia.Poult Sci. 2019 Dec 1;98(12):6527-6532. doi: 10.3382/ps/pez497. Poult Sci. 2019. PMID: 31433842 Free PMC article. Review.
-
Tibial dyschondroplasia is highly associated with suppression of tibial angiogenesis through regulating the HIF-1α/VEGF/VEGFR signaling pathway in chickens.Sci Rep. 2017 Aug 22;7(1):9089. doi: 10.1038/s41598-017-09664-6. Sci Rep. 2017. PMID: 28831181 Free PMC article.
-
Avian tibial dyschondroplasia: a morphological and biochemical review of the growth plate lesion and its causes.Vet Pathol. 1994 Jul;31(4):403-4. doi: 10.1177/030098589403100401. Vet Pathol. 1994. PMID: 7941228 Review.
Cited by
-
Abnormal Lipid Profile in Fast-Growing Broilers With Spontaneous Femoral Head Necrosis.Front Physiol. 2021 Jun 14;12:685968. doi: 10.3389/fphys.2021.685968. eCollection 2021. Front Physiol. 2021. PMID: 34194339 Free PMC article.
-
Homeostatic Regulation of Pro-Angiogenic and Anti-Angiogenic Proteins via Hedgehog, Notch Grid, and Ephrin Signaling in Tibial Dyschondroplasia.Animals (Basel). 2023 Dec 5;13(24):3750. doi: 10.3390/ani13243750. Animals (Basel). 2023. PMID: 38136788 Free PMC article. Review.
-
Pesticide thiram exposure alters the gut microbial diversity of chickens.Front Microbiol. 2022 Sep 8;13:966224. doi: 10.3389/fmicb.2022.966224. eCollection 2022. Front Microbiol. 2022. PMID: 36160266 Free PMC article.
-
Effects of Probiotics and Gut Microbiota on Bone Metabolism in Chickens: A Review.Metabolites. 2022 Oct 20;12(10):1000. doi: 10.3390/metabo12101000. Metabolites. 2022. PMID: 36295902 Free PMC article. Review.
-
Morinda officinalis Polysaccharides Ameliorates Bone Growth by Attenuating Oxidative Stress and Regulating the Gut Microbiota in Thiram-Induced Tibial Dyschondroplasia Chickens.Metabolites. 2022 Oct 10;12(10):958. doi: 10.3390/metabo12100958. Metabolites. 2022. PMID: 36295860 Free PMC article.
References
-
- Pelicia K, et al. Evaluation of a radiographic method to detect tibial dyschondroplasia lesions in broilers. Braz J Poultry Sci. 2012;14:129–135.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

