Potential Mechanisms Underlying Centralized Pain and Emerging Therapeutic Interventions
- PMID: 29487504
- PMCID: PMC5816755
- DOI: 10.3389/fncel.2018.00035
Potential Mechanisms Underlying Centralized Pain and Emerging Therapeutic Interventions
Abstract
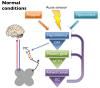
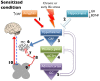
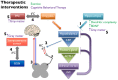
Centralized pain syndromes are associated with changes within the central nervous system that amplify peripheral input and/or generate the perception of pain in the absence of a noxious stimulus. Examples of idiopathic functional disorders that are often categorized as centralized pain syndromes include fibromyalgia, chronic pelvic pain syndromes, migraine, and temporomandibular disorder. Patients often suffer from widespread pain, associated with more than one specific syndrome, and report fatigue, mood and sleep disturbances, and poor quality of life. The high degree of symptom comorbidity and a lack of definitive underlying etiology make these syndromes notoriously difficult to treat. The main purpose of this review article is to discuss potential mechanisms of centrally-driven pain amplification and how they may contribute to increased comorbidity, poorer pain outcomes, and decreased quality of life in patients diagnosed with centralized pain syndromes, as well as discuss emerging non-pharmacological therapies that improve symptomology associated with these syndromes. Abnormal regulation and output of the hypothalamic-pituitary-adrenal (HPA) axis is commonly associated with centralized pain disorders. The HPA axis is the primary stress response system and its activation results in downstream production of cortisol and a dampening of the immune response. Patients with centralized pain syndromes often present with hyper- or hypocortisolism and evidence of altered downstream signaling from the HPA axis including increased Mast cell (MC) infiltration and activation, which can lead to sensitization of nearby nociceptive afferents. Increased peripheral input via nociceptor activation can lead to "hyperalgesic priming" and/or "wind-up" and eventually to central sensitization through long term potentiation in the central nervous system. Other evidence of central modifications has been observed through brain imaging studies of functional connectivity and magnetic resonance spectroscopy and are shown to contribute to the widespreadness of pain and poor mood in patients with fibromyalgia and chronic urological pain. Non-pharmacological therapeutics, including exercise and cognitive behavioral therapy (CBT), have shown great promise in treating symptoms of centralized pain.
Keywords: central sensitization; cognitive behavioral therapy; exercise; hypothalamic-pituitary-adrenal (HPA) axis; mast cells; pain; stress.
Figures
References
-
- ACS (2016). Cancer Facts and Figures. Atlanta, GA: American Cancer Society.
-
- ADA (2016). Statistics About Diabetes. Arlington, VA: American Diabetes Association.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

