HERVs New Role in Cancer: From Accused Perpetrators to Cheerful Protectors
- PMID: 29487579
- PMCID: PMC5816757
- DOI: 10.3389/fmicb.2018.00178
HERVs New Role in Cancer: From Accused Perpetrators to Cheerful Protectors
Abstract
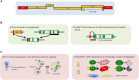
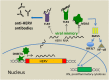
Initial indications that retroviruses are connected to neoplastic transformation were seen more than a century ago. This concept has also been tested for endogenized retroviruses (ERVs) that are abundantly expressed in many transformed cells. In healthy cells, ERV expression is commonly prevented by DNA methylation and other epigenetic control mechanisms. ERVs are remnants of former exogenous forms that invaded the germ line of the host and have since been vertically transmitted. Several examples of ERV-induced genomic recombination events and dysregulation of cellular genes that contribute to tumor formation have been well documented. Moreover, evidence is accumulating that certain ERV proteins have oncogenic properties. In contrast to these implications for supporting cancer induction, a recent string of papers has described favorable outcomes of increasing human ERV (HERV) RNA and DNA abundance by treatment of cancer cells with methyltransferase inhibitors. Analogous to an infecting agent, the ERV-derived nucleic acids are sensed in the cytoplasm and activate innate immune responses that drive the tumor cell into apoptosis. This "viral mimicry" induced by epigenetic drugs might offer novel therapeutic approaches to help target cancer cells that are normally difficult to treat using standard chemotherapy. In this review, we discuss both the detrimental and the new beneficial role of HERV reactivation in terms of its implications for cancer.
Keywords: DNA-methylation; HERV; HERV-K; cancer; human endogenous retrovirus (HERV); innate sensing; viral mimicry.
Figures
References
-
- Armbruester V., Sauter M., Krautkraemer E., Meese E., Kleiman A., Best B., et al. (2002). A novel gene from the human endogenous retrovirus K expressed in transformed cells. Clin. Cancer Res. 8 1800–1807. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

