Loss of Rictor in Monocyte/Macrophages Suppresses Their Proliferation and Viability Reducing Atherosclerosis in LDLR Null Mice
- PMID: 29487597
- PMCID: PMC5816794
- DOI: 10.3389/fimmu.2018.00215
Loss of Rictor in Monocyte/Macrophages Suppresses Their Proliferation and Viability Reducing Atherosclerosis in LDLR Null Mice
Abstract
Background: Rictor is an essential component of mammalian target of rapamycin (mTOR) complex 2 (mTORC2), a conserved serine/threonine kinase that may play a role in cell proliferation, survival and innate or adaptive immune responses. Genetic loss of Rictor inactivates mTORC2, which directly activates Akt S473 phosphorylation and promotes pro-survival cell signaling and proliferation.
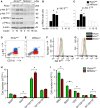
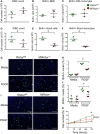
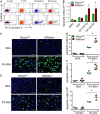
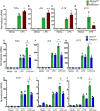
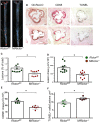
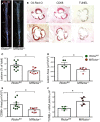
Methods and results: To study the role of mTORC2 signaling in monocytes and macrophages, we generated mice with myeloid lineage-specific Rictor deletion (MRictor-/-). These MRictor-/- mice exhibited dramatic reductions of white blood cells, B-cells, T-cells, and monocytes but had similar levels of neutrophils compared to control Rictor flox-flox (Rictorfl/fl) mice. MRictor-/- bone marrow monocytes and peritoneal macrophages expressed reduced levels of mTORC2 signaling and decreased Akt S473 phosphorylation, and they displayed significantly less proliferation than control Rictorfl/fl cells. In addition, blood monocytes and peritoneal macrophages isolated from MRictor-/- mice were significantly more sensitive to pro-apoptotic stimuli. In response to LPS, MRictor-/- macrophages exhibited the M1 phenotype with higher levels of pro-inflammatory gene expression and lower levels of Il10 gene expression than control Rictorfl/fl cells. Further suppression of LPS-stimulated Akt signaling with a low dose of an Akt inhibitor, increased inflammatory gene expression in macrophages, but genetic inactivation of Raptor reversed this rise, indicating that mTORC1 mediates this increase of inflammatory gene expression. Next, to elucidate whether mTORC2 has an impact on atherosclerosis in vivo, female and male Ldlr null mice were reconstituted with bone marrow from MRictor-/- or Rictorfl/fl mice. After 10 weeks of the Western diet, there were no differences between the recipients of the same gender in body weight, blood glucose or plasma lipid levels. However, both female and male MRictor-/- → Ldlr-/- mice developed smaller atherosclerotic lesions in the distal and proximal aorta. These lesions contained less macrophage area and more apoptosis than lesions of control Rictorfl/fl → Ldlr-/- mice. Thus, loss of Rictor and, consequently, mTORC2 significantly compromised monocyte/macrophage survival, and this markedly diminished early atherosclerosis in Ldlr-/- mice.
Conclusion: Our results demonstrate that mTORC2 is a key signaling regulator of macrophage survival and its depletion suppresses early atherosclerosis.
Keywords: Akt signaling; apoptosis; atherosclerosis; macrophages; mammalian target of rapamycin complex 2; monocytes; proliferation.
Figures
References
-
- Linton MF, Yancey PG, Davies SS, Jerome WGJ, Linton EF, Vickers KC. The role of lipids and lipoproteins in atherosclerosis. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDTextcom, Inc. (2000). p. 1–92. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343489/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

