Dectin-1-Syk-CARD9 Signaling Pathway in TB Immunity
- PMID: 29487599
- PMCID: PMC5816931
- DOI: 10.3389/fimmu.2018.00225
Dectin-1-Syk-CARD9 Signaling Pathway in TB Immunity
Abstract
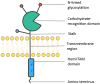
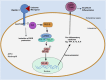
One of the first steps toward mounting an effective immune response to Mycobacterium tuberculosis (Mtb) is recognition of the pathogen through pattern-recognition receptors (PRRs) expressed by innate immune cells. Activation of the PRR Dectin-1 by an unknown mycobacterial ligand triggers an intracellular signaling cascade involving numerous proteins, including spleen tyrosine kinase, protein kinase C-delta, and caspase recruitment domain family member 9, some of which have been shown to influence host immune response to TB infection. Here, we review the role of Dectin-1 signaling pathway in anti-mycobacterial immunity and discuss its contribution in the control of Mtb infection, and potential applications in TB vaccine adjuvanticity.
Keywords: C-type lectin receptors; Mycobacterium tuberculosis; TB immunity; dectin-1; innate immunity; pathogen-associated molecular patterns; pattern-recognition receptors; tuberculosis.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

