A 10-Year Prospective Study of Implant-Based Breast Augmentation and Reconstruction
- PMID: 29487671
- PMCID: PMC5807774
A 10-Year Prospective Study of Implant-Based Breast Augmentation and Reconstruction
Abstract
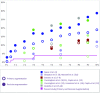
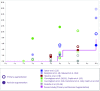
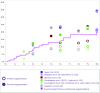
Objective: Observational studies are essential for ensuring patient safety, decreasing complications, and developing better surgical techniques and implants. The primary objective of this study is to demonstrate the safety and efficacy of Sebbin breast implants in both augmentation and reconstruction cohorts. Methods: This prospective, multicenter, observational 10-year study conducted in France included 205 patients (385 implants) who underwent breast augmentation (n = 166) or reconstruction (n = 39) with Sebbin round silicone gel implants. Data on patient demographics, surgical details, and complications were collected. Results: Median patient age was 39 years; 20.5% of patients were smokers. The augmentation cohort included 166 patients (81.0%); the reconstruction cohort, 39 patients (19.0%). Median implant volume was 280 ml; 91.2% of implants were textured, and 8.8% were smooth. Average patient follow-up was 63 months. The most frequent surgical approach in the Augmentation Cohort was periareolar (72.4%), with 45.5% submuscular and 51.5% subglandular placements. All patients received antibiotic prophylaxis, and postoperative antibiotic therapy was given to 39.5% of patients (average 4.8 days). Drainage was performed in 59.5% of patients (average 2.9 days). Of the reconstruction cohort, 64.1% had preoperative radiotherapy. Nine patients had Baker III/IV capsular contracture (3 bilateral; 4 had a history of radiotherapy) and 7 patients had implant rupture; 41 patients underwent explantation. No cases of double capsule, late seroma, or anaplastic large cell lymphoma occurred. Conclusions: This study found an excellent safety profile and very low capsular contracture rate with breast augmentation and reconstruction using Sebbin round silicone gel implants.
Keywords: Breast implant; breast surgery; capsular contracture; cohort study; texturation.
Figures


References
-
- ISAPS global statistics: ISAPS International Survey on Aesthetic/Cosmetic Procedures Performed in 2015. Hanover, NH: International Society of Aesthetic Plastic Surgery; 2015. International Society of Aesthetic Plastic Surgery. www.isaps.org.
-
- Spear SL, Murphy DK, Slicton A, Walker PS. Inamed silicone breast implant USSG. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(7) suppl 1:8S–16S. discussion 7S-8S. - PubMed
-
- Bengtson BP, Van Natta BW, Murphy DK, Slicton A, Maxwell GP. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plast Reconstr Surg. 2007;120(7) suppl 1:40S–8S. - PubMed
-
- Maxwell GP, Van Natta BW, Murphy DK, Slicton A, Bengtson BP. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;32(6):709–17. - PubMed
LinkOut - more resources
Full Text Sources
