Mutations in DNA repair genes are associated with increased neo-antigen load and activated T cell infiltration in lung adenocarcinoma
- PMID: 29487705
- PMCID: PMC5814272
- DOI: 10.18632/oncotarget.23742
Mutations in DNA repair genes are associated with increased neo-antigen load and activated T cell infiltration in lung adenocarcinoma
Abstract
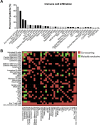
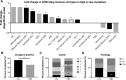
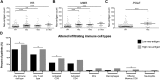
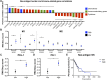
Mutations in DNA repair genes lead to increased genomic instability and mutation frequency. These mutations represent potential biomarkers for cancer immunotherapy efficacy, as high tumor mutational burden has been associated with increased neo-antigens and tumor infiltrating lymphocytes. While mismatch repair mutations have successfully predicted response to anti-PD-1 therapy in colorectal and other cancers, they have not yet been tested for lung cancer, and few have investigated genes from other DNA repair pathways. We utilized TCGA samples to comprehensively immunophenotype lung tumors and analyze the links between DNA repair mutations, neo-antigen and total mutational burden, and tumor immune infiltration. Overall, 73% of lung tumors contained infiltration by at least one T cell subset, with high mutational burden tumors containing significantly increased infiltration by activated CD4 and CD8 T cells. Further, mutations in mismatch repair genes, homologous recombination genes, or POLE accurately predicted increased tumor mutational burden, neo-antigen load, and T cell infiltration. Finally, neo-antigen load correlated with expression of M1-polarized macrophage genes, PD-1, PD-L1, IFNγ, GZMB, and FASLG, among other immune-related genes. Overall, after defining the immune infiltrate in lung tumors, we demonstrate the potential value of utilizing gene mutations from multiple DNA repair pathways as biomarkers for lung cancer immunotherapy.
Keywords: DNA repair; lung cancer; neo-antigens; tumor infiltrating lymphocytes; tumor mutational burden.
Conflict of interest statement
CONFLICTS OF INTEREST All authors certify that they have no conflicts of interest to disclose.
Figures

References
-
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. https://doi.org/10.1038/nature12477 - DOI - PMC - PubMed
-
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. https://doi.org/10.1056/NEJMoa1305133 - DOI - PMC - PubMed
-
- Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, Reck M. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–54. https://doi.org/10.1200/JCO.2011.38.4032 - DOI - PubMed
-
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. https://doi.org/10.1073/pnas.1533209100 - DOI - PMC - PubMed
-
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. https://doi.org/10.1056/NEJMoa1501824 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

