Effect of Feeding Bacillus subtilis Spores to Broilers Challenged with Salmonella enterica serovar Heidelberg Brazilian Strain UFPR1 on Performance, Immune Response, and Gut Health
- PMID: 29487856
- PMCID: PMC5816941
- DOI: 10.3389/fvets.2018.00013
Effect of Feeding Bacillus subtilis Spores to Broilers Challenged with Salmonella enterica serovar Heidelberg Brazilian Strain UFPR1 on Performance, Immune Response, and Gut Health
Abstract
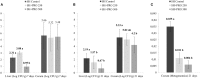
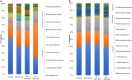
Salmonellosis is a poultry industry and public health concern worldwide. Recently, Salmonella enterica serovar Heidelberg (SH) has been reported in broilers in Brazil. The effect of feeding a blend of three strains of Bacillus subtilis (PRO) was studied in broilers orally challenged (107 CFU/chick) or not with a SH isolated in south of Brazil (UFPR1 strain). Twelve male Cobb 500 broilers per pen were randomly assigned to six treatments in a 3 × 2 factorial experiment where PRO was added at 0, 250, or 500 g/ton of broiler feed and fed to either SH-challenged (SH Control, SH + PRO 250, and SH + PRO 500) or non-challenged birds (Control, PRO 250, and PRO 500). Broiler performance, histologic alterations in intestinal morphology, Salmonella quantification and immune cells counts in liver (macrophages, T CD4+ and T CD8+) were analyzed. Changes in the intestinal microbiota of broilers were also studied by metagenomics for Control, SH Control, SH + PRO 250, and SH + PRO 500 only. Feeding PRO at 250 or 500 g/ton reduced SH counts and incidence in liver and cecum at 21 days of age. It was observed that PRO groups increased the macrophage mobilization to the liver in SH-challenged birds (P < 0.05) but reduced these cells in the liver of non-challenged birds, showing an interesting immune cell dynamics effect. PRO at 250 g/ton did not affect gut histology, but improved animal performance (P < 0.05) while PRO at 500/ton did not affect animal performance but increased histologic alteration related to activation of the defense response in the ileum in SH challenged birds compared to control birds (P < 0.05). SH + PRO 500 group presented a more diverse cecal microbiota (Shannon-Wiener index; P < 0.05) compared to Control and SH Control groups; while SH + PRO 250 had greater ileal richness (JackkNife index) compared to Control (P < 0.05). PRO was effective in reducing Salmonella colonization in liver and cecum when fed at 250 or 500 g/ton to broilers inoculated with SH strain UFPR1. PRO promotes positive alterations in performance (at 250 g/ton), immune modulatory effect in the gastrointestinal tract, SH reduction, and intestinal microbiota modulation.
Keywords: 16S; gut health; gut microbiome; immunity; poultry; probiotic; salmonellosis.
Figures




References
-
- Carter AJ, Adams MR, Woodward MJ, La Ragione RM. Control strategies for Salmonella colonization of poultry: the probiotic perspective. Food Sci Technol (2009) 5:103–15.
-
- Centers for Disease Control and Prevention. National Antimicrobial Resistance Monitoring System: Enteric Bacteria. Human Isolates Final Report. Atlanta: The Centers; (2014).
-
- Hoffmann M, Zhao S, Pettengill J, Luo Y, Monday SR, Abbott J, et al. Comparative genomic analysis and virulence differences in closely related Salmonella enterica serotype Heidelberg isolates from humans, retail meats, and animals. Genome Biol Evol (2014) 6:1046–68. 10.1093/gbe/evu079 - DOI - PMC - PubMed
-
- Santin E, Hayashi RM, Wammes JC, Gonzales-Esquerra R, Carazzolle MF, Freire CCM, et al. Phenotypic and genotypic features of a Salmonella Heidelberg strain isolated in broilers in Brazil and their possible association to antibiotics and short-chain organic acids resistance and susceptibility. Front Vet Sci (2017) 4:184. 10.3389/fvets.2017.00184 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

