Mechanisms of antifreeze proteins investigated via the site-directed spin labeling technique
- PMID: 29487966
- PMCID: PMC6709975
- DOI: 10.1007/s00249-018-1285-3
Mechanisms of antifreeze proteins investigated via the site-directed spin labeling technique
Abstract
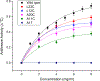
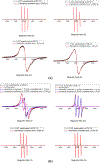
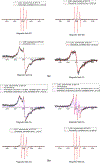
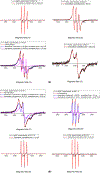
The site-directed spin labeling (SDSL) technique was used to examine the antifreeze mechanisms of type-I antifreeze proteins (AFPs). The effects on the growth of seed ice crystals by the spin-label groups attached to different side chains of the AFPs were observed, and the states of water molecules surrounding the spin-label groups were probed via analyses of variable-temperature (VT) dependent electron paramagnetic resonance (EPR) spectra. The first set of experiments revealed the antifreeze activities of the spin-labeled AFPs at the microscopic level, while the second set of experiments displayed those at the molecular level. The experimental results confirmed the putative ice-binding surface (IBS) of type-I AFPs. The VT EPR spectra indicate that type-I AFPs can inhibit the nucleation of seed ice crystals down to ~ - 20 °C in their aqueous solutions. Thus, the present authors believe that AFPs protect organisms from freezing damage in two ways: (1) inhibiting the nucleation of seed ice crystals, and (2) hindering the growth of seed ice crystals once they have formed. The first mechanism should play a more significant role in protecting against freezing damage among organisms living in cold environments. The VT EPR spectra also revealed that liquid-like water molecules existed around the spin-labeled non-ice-binding side chains of the AFPs frozen within the ice matrices, and ice surrounding the spin-label groups melted at subzero temperatures during the heating process. This manuscript concludes with the proposed model of antifreeze mechanisms of AFPs based on the experimental results.
Keywords: Ice crystals; Ice growth inhibition; Ice nucleation inhibition; Site-directed spin labeling; Type-I antifreeze protein; VT EPR.
Conflict of interest statement
Figures








References
-
- Altenbach C (2018) Multi-component EPR fitting version 742, Lab-VIEW software. From California https://sites.google.com/site/altenbach/labview-programs/epr-programs/mu...
-
- Ba Y, Wongskhaluang J (2003) Reversible binding of the HPLC6 isoform of type I antifreeze proteins to ice surfaces and the antifreeze mechanism studied by multiple quantum (MQ) filtering–spin exchange NMR experiment. J Am Chem Soc 125(2):330–331 - PubMed
-
- Calvaresi M, Hӧfinger S (2012) Local ice melting by an antifreeze protein. Biomacromol 13:2046–2052 - PubMed
-
- Chao HLM (1994) Structure–function relationships in type III anti-freeze protein (cell lysis, ice recrystallization) Copyright (C) 2011 American Chemical Society (ACS) All Rights Reserved
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

