Multi-centre phase IV trial to investigate the immunogenicity of a new liquid formulation of recombinant human growth hormone in adults with growth hormone deficiency
- PMID: 29488103
- PMCID: PMC6061248
- DOI: 10.1007/s40618-017-0818-4
Multi-centre phase IV trial to investigate the immunogenicity of a new liquid formulation of recombinant human growth hormone in adults with growth hormone deficiency
Abstract
Purpose: To investigate whether a new liquid formulation of recombinant human growth hormone (r-hGH) induces the production of binding antibodies (BAbs) in adults with congenital or adult-onset growth hormone deficiency (GHD).
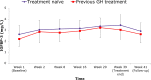
Methods: Men or women aged 19-65 years with adult growth hormone deficiency who were r-hGH-naïve or had stopped treatment ≥ 1 month before screening were treated with between 0.15 and 0.30 mg/day r-hGH liquid formulation for 39 weeks. The primary endpoint was the proportion of patients who developed BAbs at any time. Secondary endpoints were the proportion of patients with BAbs who became positive for neutralising antibodies, the effects on biomarkers of r-hGH exposure, safety, and adherence to treatment downloaded from the easypod™ connect software.
Results: Seventy-eight patients (61.5% men) with mean age 44.5 years (range 21-65) started and 68 (87.2%) completed the 39-week treatment period. 82.1% were treatment naïve; all were negative for BAbs to r-hGH at baseline. The median (interquartile range) duration of treatment [273 (267.0-277.0) days] was consistent with patients receiving the required doses, and mean treatment adherence measured using easypod™ connect was 89.3%. The proportion of patients who developed BAbs was 0% (95% confidence interval 0-4.68%) and biomarker profiles were consistent with exposure to r-hGH. 92.3% of patients reported ≥ 1 adverse event during treatment. Most events were mild or moderate and no new safety concerns were detected.
Conclusions: The low immunogenicity profile of the liquid formulation was consistent with that for the freeze-dried formulation, and no new safety concerns were reported.
Keywords: Adult growth hormone deficiency; Binding antibodies; Liquid formulation; Neutralising antibodies; Recombinant human growth hormone.
Conflict of interest statement
Conflict of interest
GJ has received speaker’s honoraria and grants from Novartis, Novo Nordisk, Pfizer and Sandoz, speaker’s honoraria from Merck Serono and Otsuka, and consultancy fees from Astra Zeneca and Shire. KN is an employee of Merck KGaA, Darmstadt, Germany. VA is an employee of EMD Serono, a business of Merck KGaA, Darmstadt, Germany. MM has no conflicts of interest. UP has no conflicts of interest.
Ethical approval
All procedures performed in this study involving human participants were approved by the institutional and/or national research ethics committee and complied with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures


References
-
- Nbul MM. Evaluation of short and tall stature in children. Am Fam Phys. 2008;78:597–604. - PubMed
-
- Whitehead HM, Boreham C, McIlrath EM, Sheridan B, Kennedy L, Atkinson AB, Hadden DR. Growth hormone treatment of adults with growth hormone deficiency: results of a 13-month placebo controlled cross-over study. Clin Endocrinol (Oxf) 1992;36(1):45–52. doi: 10.1111/j.1365-2265.1992.tb02901.x. - DOI - PubMed
-
- NICE (2010) Human growth hormone for the treatment of growth failure in children. London: NICE
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

