Neuroimaging of the Injured Pediatric Brain: Methods and New Lessons
- PMID: 29488436
- PMCID: PMC6070434
- DOI: 10.1177/1073858418759489
Neuroimaging of the Injured Pediatric Brain: Methods and New Lessons
Abstract
Traumatic brain injury (TBI) is a significant public health problem in the United States, especially for children and adolescents. Current epidemiological data estimate over 600,000 patients younger than 20 years are treated for TBI in emergency rooms annually. While many patients experience a full recovery, for others there can be long-lasting cognitive, neurological, psychological, and behavioral disruptions. TBI in youth can disrupt ongoing brain development and create added family stress during a formative period. The neuroimaging methods used to assess brain injury improve each year, providing researchers a more detailed characterization of the injury and recovery process. In this review, we cover current imaging methods used to quantify brain disruption post-injury, including structural magnetic resonance imaging (MRI), diffusion MRI, functional MRI, resting state fMRI, and magnetic resonance spectroscopy (MRS), with brief coverage of other methods, including electroencephalography (EEG), single-photon emission computed tomography (SPECT), and positron emission tomography (PET). We include studies focusing on pediatric moderate-severe TBI from 2 months post-injury and beyond. While the morbidity of pediatric TBI is considerable, continuing advances in imaging methods have the potential to identify new treatment targets that can lead to significant improvements in outcome.
Keywords: DTI; MRI; MRS; brain imaging; cognitive; fMRI; longitudinal; multimodal; pediatric; traumatic brain injury.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
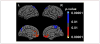
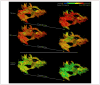

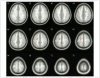
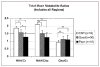

References
-
- Acerini CL, Tasker RC. Traumatic brain injury induced hypothalamic-pituitary dysfunction: a paediatric perspective. Pituitary. 2007;10:373–80. - PubMed
-
- Anderson V, Brown S, Newitt H, Hoile H. Educational, vocational, psychosocial, and quality-of-life outcomes for adult survivors of childhood traumatic brain injury. J Head Trauma Rehabil. 2009a;24:303–12. - PubMed
-
- Anderson V, Catroppa C. Memory outcome at 5 years post-childhood traumatic brain injury. Brain Inj. 2007;21:1399–409. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

