Neuronal Cell Death
- PMID: 29488822
- PMCID: PMC5966715
- DOI: 10.1152/physrev.00011.2017
Neuronal Cell Death
Abstract
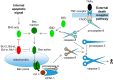
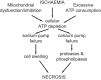
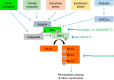
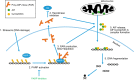
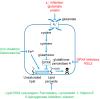
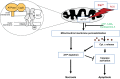
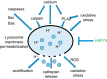
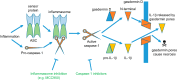
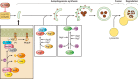
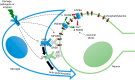
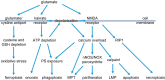
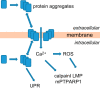
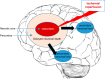
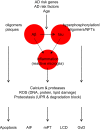
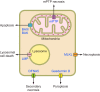
Neuronal cell death occurs extensively during development and pathology, where it is especially important because of the limited capacity of adult neurons to proliferate or be replaced. The concept of cell death used to be simple as there were just two or three types, so we just had to work out which type was involved in our particular pathology and then block it. However, we now know that there are at least a dozen ways for neurons to die, that blocking a particular mechanism of cell death may not prevent the cell from dying, and that non-neuronal cells also contribute to neuronal death. We review here the mechanisms of neuronal death by intrinsic and extrinsic apoptosis, oncosis, necroptosis, parthanatos, ferroptosis, sarmoptosis, autophagic cell death, autosis, autolysis, paraptosis, pyroptosis, phagoptosis, and mitochondrial permeability transition. We next explore the mechanisms of neuronal death during development, and those induced by axotomy, aberrant cell-cycle reentry, glutamate (excitoxicity and oxytosis), loss of connected neurons, aggregated proteins and the unfolded protein response, oxidants, inflammation, and microglia. We then reassess which forms of cell death occur in stroke and Alzheimer's disease, two of the most important pathologies involving neuronal cell death. We also discuss why it has been so difficult to pinpoint the type of neuronal death involved, if and why the mechanism of neuronal death matters, the molecular overlap and interplay between death subroutines, and the therapeutic implications of these multiple overlapping forms of neuronal death.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

