Application of seminal plasma to female genital tract prior to embryo transfer in assisted reproductive technology cycles (IVF, ICSI and frozen embryo transfer)
- PMID: 29489026
- PMCID: PMC6491093
- DOI: 10.1002/14651858.CD011809.pub2
Application of seminal plasma to female genital tract prior to embryo transfer in assisted reproductive technology cycles (IVF, ICSI and frozen embryo transfer)
Abstract
Background: The female genital tract is not exposed to seminal plasma during standard assisted reproductive technology (ART) cycles. However, it is thought that the inflammatory reaction triggered by seminal plasma may be beneficial by inducing maternal tolerance to paternal antigens expressed by the products of conception, and may increase the chance of successful implantation and live birth.
Objectives: To assess the effectiveness and safety of application of seminal plasma to the female genital tract prior to embryo transfer in ART cycles.
Search methods: We searched the following databases from inception to October 2017: Cochrane Gynaecology and Fertility Group Specialised Register of Controlled Trials, Cochrane Central Register of Studies Online (CRSO), MEDLINE, Embase, CINAHL and PsycINFO. We also searched trial registers for ongoing trials, including International Clinical Trials Registry Platform (ICTRP) Search Portal and ClinicalTrials.gov. Other sources searched were; Web of Knowledge, OpenGrey, LILACS, PubMed, Google Scholar and the reference lists of relevant articles.
Selection criteria: We included randomised controlled trials (RCTs) conducted among women undergoing ART, comparing any procedure that would expose the female genital tract to seminal plasma during the period starting five days before embryo transfer and ending two days after it versus no seminal plasma application.
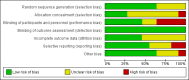
Data collection and analysis: Two review authors independently selected trials, assessed risk of bias, and extracted data. We pooled data to calculate relative risks (RRs) and 95% confidence intervals (CIs). We assessed statistical heterogeneity using the I2 statistic. We assessed the overall quality of the evidence for the main outcomes using GRADE methods. Our primary outcomes were live birth rate and miscarriage rate. Secondary outcomes were live birth/ongoing pregnancy rate, clinical pregnancy rate, multiple pregnancy rate, ectopic pregnancy rate and the incidence of other adverse events.
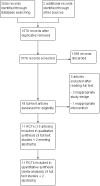
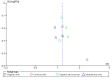
Main results: We included 11 RCTs (3215 women). The quality of the evidence ranged from very low to low. The main limitations were risk of bias (associated with poor reporting of allocation concealment and other methods) and imprecision for the primary outcome of live birth rate.Live birth rates: There was insufficient evidence to determine whether there was a difference between the groups with respect to live birth rates (RR 1.10, 95% CI 0.86 to 1.43; participants = 948; studies = 3; I2 = 0%). Low quality evidence suggests that if the live birth rate following standard ART is 19% it will be between 16% and 27% with seminal plasma application.Miscarriage rate: There was insufficient evidence to determine whether there was a difference between the groups (RR 1.01, 95% CI 0.57 to 1.79; participants = 1209; studies = 4; I2 = 0%). Low quality evidence suggests that if the miscarriage rate following standard ART is 3.7%, the miscarriage rate following seminal plasma application will be between 2.1% and 6.6%.Live birth or ongoing pregnancy rates: Seminal plasma application makes little or no difference in live birth or ongoing pregnancy rates (RR 1.19, 95% CI 0.95 to 1.49; participants = 1178; studies = 4; I2 = 4%, low quality evidence). The evidence suggests that if the live birth or ongoing pregnancy rate following standard ART is 19.5% it will be between 18.5% and 29% with seminal plasma application.Clinical pregnancy rates: Seminal plasma application may increase clinical pregnancy rates (RR 1.15, 95% CI 1.01 to 1.31; participants = 2768; studies = 10; I2 = 0%). Very low quality evidence suggests that if the clinical pregnancy rate following standard ART is 22.0% it will be between 22.2% and 28.8% with seminal plasma application. This finding should be regarded with caution, as a post-hoc sensitivity analysis restricted to studies at overall low risk of bias did not find a significant difference between the groups (RR 1.06, 95% CI 0.81 to 1.39; participants = 547; studies = 3; I2 = 0%).Multiple pregnancy rate: Seminal plasma application may make little or no difference to multiple pregnancy rates (RR 1.11, 95% CI 0.76 to 1.64; participants = 1642; studies = 5; I2 = 9%). Low quality evidence suggests that if the multiple pregnancy rate following standard ART is 7%, the multiple pregnancy rate following seminal plasma application will be between 5% and 11.4%.Ectopic pregnancy: There was insufficient evidence to determine whether seminal plasma application influences the risk of ectopic pregnancy (RR 1.59, 95% CI 0.20 to 12.78, participants =1521; studies = 5; I2 = 0%) .Infectious complications or other adverse events: No data were available on these outcomes AUTHORS' CONCLUSIONS: In women undergoing ART, there was insufficient evidence to determine whether there was a difference between the seminal plasma and the standard ART group in rates of live birth (low-quality evidence) or miscarriage (low quality evidence). There was low quality evidence suggesting little or no difference between the groups in rates of live birth or ongoing pregnancy (composite outcome). We found low quality evidence that seminal plasma application may be associated with more clinical pregnancies than standard ART. There was low quality evidence suggesting little or no difference between the groups in rates of multiple pregnancy. There was insufficient evidence to reach any conclusions about the risk of ectopic pregnancy, and no data were available on infectious complications or other adverse events.We conclude that seminal plasma application is worth further investigation, focusing on live birth and miscarriage rates.
Conflict of interest statement
WB, AS and AMAS have no interests to declare.
BA's institution has received fees for a consultancy, two lectures and a conference registration, all relating to contraception.
Figures




Update of
- doi: 10.1002/14651858.CD011809
Similar articles
-
Day three versus day two embryo transfer following in vitro fertilization or intracytoplasmic sperm injection.Cochrane Database Syst Rev. 2016 Dec 14;12(12):CD004378. doi: 10.1002/14651858.CD004378.pub3. Cochrane Database Syst Rev. 2016. PMID: 27976360 Free PMC article.
-
Recombinant luteinizing hormone (rLH) and recombinant follicle stimulating hormone (rFSH) for ovarian stimulation in IVF/ICSI cycles.Cochrane Database Syst Rev. 2017 May 24;5(5):CD005070. doi: 10.1002/14651858.CD005070.pub3. Cochrane Database Syst Rev. 2017. PMID: 28537052 Free PMC article.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD007807. doi: 10.1002/14651858.CD007807.pub4. PMID: 28752910 Free PMC article. Updated.
-
Time-lapse systems for embryo incubation and assessment in assisted reproduction.Cochrane Database Syst Rev. 2018 May 25;5(5):CD011320. doi: 10.1002/14651858.CD011320.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2019 May 29;5:CD011320. doi: 10.1002/14651858.CD011320.pub4. PMID: 29800485 Free PMC article. Updated.
-
Metabolomics for improving pregnancy outcomes in women undergoing assisted reproductive technologies.Cochrane Database Syst Rev. 2018 Mar 16;3(3):CD011872. doi: 10.1002/14651858.CD011872.pub3. Cochrane Database Syst Rev. 2018. PMID: 29547689 Free PMC article.
Cited by
-
Good practice recommendations on add-ons in reproductive medicine†.Hum Reprod. 2023 Nov 2;38(11):2062-2104. doi: 10.1093/humrep/dead184. Hum Reprod. 2023. PMID: 37747409 Free PMC article.
-
Seminal Plasma: Relevant for Fertility?Int J Mol Sci. 2021 Apr 22;22(9):4368. doi: 10.3390/ijms22094368. Int J Mol Sci. 2021. PMID: 33922047 Free PMC article. Review.
-
Immune regulatory cytokines in seminal plasma of healthy men: A scoping review and analysis of variance.Andrology. 2023 Oct;11(7):1245-1266. doi: 10.1111/andr.13424. Epub 2023 Apr 10. Andrology. 2023. PMID: 36891953 Free PMC article.
-
Endometrial Immune Dysfunction in Recurrent Pregnancy Loss.Int J Mol Sci. 2019 Oct 26;20(21):5332. doi: 10.3390/ijms20215332. Int J Mol Sci. 2019. PMID: 31717776 Free PMC article. Review.
-
Assisted reproductive technology: an overview of Cochrane Reviews.Cochrane Database Syst Rev. 2018 Aug 17;8(8):CD010537. doi: 10.1002/14651858.CD010537.pub5. Cochrane Database Syst Rev. 2018. PMID: 30117155 Free PMC article.
References
References to studies included in this review
Aflatoonian 2009 {published data only (unpublished sought but not used)}
-
- Aflatoonian A, Ghandi S, Tabibnejad N. The effect of intercourse around embryo transfer on pregnancy rate in assisted reproductive technology cycles. International Journal of Fertility and Sterility 2009;2(4):169‐72.
Bellinge 1986 {published data only}
-
- Bellinge BS, Copeland CM, Thomas TD, Mazzucchelli RE, O'Neil G, Cohen MJ. The influence of patient insemination on the implantation rate in an in vitro fertilization and embryo transfer program. Fertility and Sterilty 1989; Vol. 51, issue 1:135‐8. - PubMed
Chicea 2013 {published data only (unpublished sought but not used)}
Crawford 2015 {published data only (unpublished sought but not used)}
-
- Crawford G, Tovar G, Olivier F, Dilgil M, Gudi A, Shah A, et al. The effect of intrauterine injection of seminal plasma on IVF results a prospective double‐blind randomised placebo‐controlled trial. British Journal of Obstetrics and Gynaecology 2015;122(S1):379.
Friedler 2013 {published data only (unpublished sought but not used)}
-
- Friedler S, Ben‐Ami I, Gidoni Y, Strassburger D, Kasterstein E, Maslansky B, et al. Effect of seminal plasma application to the vaginal vault in in vitro fertilization or intracytoplasmic sperm injection treatment cycles ‐ a double‐blind, placebo‐controlled, randomized study. Journal of Assisted Reproduction and Genetics 2013;30:907‐11. - PMC - PubMed
Jafarabadi 2016 {published data only (unpublished sought but not used)}
-
- Jafarabadi M, Sasani A, Ramezanzadeh F, Zandieh Z, Shariat M, Haghollahi F. Intracervical application of seminal plasma at the time of oocyte pickup during in vitro fertilization. Acta Medica Mediterranea 2016;32:2085‐90.
Karimian 2010 {published data only}
-
- Karimian L, Naghibi ZH, Yazdi PE, Moini A, Valojerdi MR, Akhondi MM, et al. The effect of intercourse on B HcG level and pregnancy outcome after IVF cycles. Reproductive Biomedicine Online 2010;20(Supplement 3):S65.
Mayer 2015 {published and unpublished data}
-
- Mayer RB, Ebner T, Yaman C, Hartl J, Sir A, Krain V, et al. Influence of intracervical and intravaginal seminal plasma on the endometrium in assisted reproduction: a double‐blind, placebo‐controlled, randomized study. Ultrasound in Obstetrics and Gynecology 2015;45:132‐8. - PubMed
-
- Mayer RB, Shebl O, Krain V, Hartl J, Oppelt P, Ebner T. The influence of intracervical and intravaginal application of seminal plasma on the endometrium and life birth rate: a prospective, double blind, placebo controlled, randomized study. Geburtshilfe und Frauenheilkunde 2013;73:P 08.
Tremellen 2000 {published data only}
-
- Tremellen KP, Valbuena D, Landeras J, Ballesteros A, Martinez J, Mendoza S, et al. The effect of intercourse on pregnancy rates during assisted human reproduction. Human Reproduction 2000; Vol. 15, issue 12:2653‐8. - PubMed
Von Wolff 2009 {published data only}
-
- Germeyer A, Rösner S, Jauckus J, Strowitzki T, Wolff M. Intrauterine application of diluted seminal plasma in in vitro fertilization does not improve pregnancy rates ‐ a placebo controlled double blind randomized trial. Human Reproduction 2013;28(Supp 1):i215. - PubMed
-
- Wolff M, Rösner S, Thöne C, Pinheiro RM, Jauckus J, Bruckner T, et al. Intravaginal and intracervical application of seminal plasma in in vitro fertilization or intracytoplasmic sperm injection treatment cycles ‐ a double‐blind, placebo‐controlled, randomized pilot study. Fertility and Sterility 2009;91(1):167‐72. - PubMed
Von Wolff 2013 {published data only (unpublished sought but not used)}
-
- Germeyer A, Rosner S, Jauckus J, Strowitzki T, Wolff M. Intrauterine application of diluted seminal plasma in in vitro fertilization does not improve pregnancy rates ‐ a placebo controlled double blinded randomized trial. Human Reproduction 2013; Vol. 28, issue Supp 1:i215 (p240). - PubMed
-
- Wolff M, Rösner S, Germeyer A, Jauckus J, Griesinger G, Strowitzki T. Intrauterine instillation of diluted seminal plasma at oocyte pick‐up does not increase the IVF pregnancy rate: a double‐blind, placebo controlled, randomized study. Human Reproduction 2013;28(12):3247‐52. - PubMed
References to studies excluded from this review
Coulam 1995 {published data only}
-
- Coulam CB, Stern J J. Effect of seminal plasma on implantation rates. Early Pregnancy 1995;1:33‐6. - PubMed
Fishel 1989 {published data only}
-
- Fishel S, Webster J, Jackson P, Faratian B. Evaluation of high vaginal insemination at oocyte recovery in patients undergoing in vitro fertilization. Fertility and Sterility 1989;51(1):135‐8. - PubMed
Lou 2014 {published data only}
-
- Lou SG, Hagshafiha M, Yekta Z, Oshnouei S, Firoozi E, Pashapoor S, et al. Effects of intravaginal application of seminal plasma on embryo implantation and early abortion rate in patients undergoing intracytoplasmic sperm injection. Iranian Journal of Obstetrics, Gynecology and Infertility 2014;17(101):6‐12.
Additional references
Achache 2006
-
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Human Reproduction Update 2006;12(6):731‐46. - PubMed
Bromfield 2014
Chen 2014
Crawford 2015b
-
- Crawford G, Ray A, Gudi A, Shah S, Homburg R. The role of seminal plasma for improved outcomes during in vitro fertilization treatment: review of the literature and meta‐analysis. Human Reproduction Update 2015;21(2):275‐84. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 3 November 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Lee 2015
-
- Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD‐A): systematic review. Human Reproduction 2015;30(2):473‐83. - PubMed
Robertson 2002
-
- Robertson SA, Ingman WV, O'Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta ‐ a mediator of immune deviation in seminal plasma. Journal of Reproductive Immunology 2002;57:109‐28. - PubMed
Robertson 2005
-
- Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell and Tissue Research 2005;322:43‐52. - PubMed
Robertson 2013
-
- Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. Seminal fluid and the generation of regulatory T cells for embryo implantation. American Journal of Reproductive Immunology 2013;69:315‐30. - PubMed
Samy 2006
-
- Samy ET, Setiady YY, Ohno K, Pramoonjago P, Sharp C, Tung KS. The role of physiological self‐antigen in the acquisition and maintenance of regulatory T‐cell function. Immunological Reviews 2006;212:170‐84. - PubMed
Sato 2003
-
- Sato K, Yamashita N, Baba M, Matsuyama T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood 2003;101:3581‐9. - PubMed
Simon 2000
-
- Simon C, Martin JC, Pellicer A. Paracrine regulators of implantation. Best Practice & Research Clinical Obstetrics & Gynaecology 2000;14(5):815‐26. - PubMed
Tafuri 1995
-
- Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science 1995;270:630‐3. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

