Confirmation of the Cardiac Safety of PGF2α Receptor Antagonist OBE022 in a First-in-Human Study in Healthy Subjects, Using Intensive ECG Assessments
- PMID: 29489066
- PMCID: PMC6221050
- DOI: 10.1002/cpdd.447
Confirmation of the Cardiac Safety of PGF2α Receptor Antagonist OBE022 in a First-in-Human Study in Healthy Subjects, Using Intensive ECG Assessments
Abstract
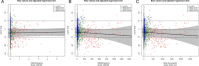
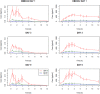
OBE022, a new orally active prostaglandin F2α receptor antagonist (OBE022) with myometrial selectivity is being developed to reduce uterine contractions during preterm labor. This first-in-human study evaluated the effect of OBE022 following multiple doses on the QT interval in 23 healthy postmenopausal women, using the effect of a meal on QTc to demonstrate assay sensitivity. We report the cardiac safety outcome performed during the multiple ascending part of this trial. OBE022 was administered after a standardized breakfast on day 1 and in the fasted state from day 3 to day 9 wth a standardized lunch 4 hours after administration. Concentration-effect modeling was used to assess the effect of prodrug OBE022 and parent OBE002 on QTc after a single dose (days 1 and 3) and multiple doses (day 9). The concentration-response analysis showed the absence of QTc prolongation at all doses tested. Two-sided 90% confidence intervals of the geometric mean Cmax for estimated QTc effects of OBE022 and OBE002 of all dose groups were consistently below the threshold of regulatory concern. The sensitivity of this study to detect small changes in the QTc was confirmed by a significant shortening of the QTc on days 1, 3, and 9 after standardized meals. This study establishes that neither prodrug OBE022 nor parent OBE002 prolong the QTc interval. The observed food effect on the QT interval validated the assay on all assessment days. Both the change from predose, premeal and the change from premeal, postdose demonstrated the specificity of the method.
Keywords: FP-receptor pharmacology; OBE022; QTc; QTc prolongation; QTc shortening; food effect; obstetrics; safety pharmacology; tocolytic.
© 2018, The Authors. Clinical Pharmacology in Drug Development published by Wiley Periodicals, Inc. on behalf of The American College of Clinical Pharmacology.
Figures


Similar articles
-
Coadministration of the prostaglandin F2α receptor antagonist preterm labour drug candidate OBE022 with magnesium sulfate, atosiban, nifedipine and betamethasone.Br J Clin Pharmacol. 2019 Jul;85(7):1516-1527. doi: 10.1111/bcp.13925. Epub 2019 May 11. Br J Clin Pharmacol. 2019. PMID: 30891820 Free PMC article. Clinical Trial.
-
Pharmacokinetics, safety and tolerability of OBE022, a selective prostaglandin F2α receptor antagonist tocolytic: A first-in-human trial in healthy postmenopausal women.Br J Clin Pharmacol. 2018 Aug;84(8):1839-1855. doi: 10.1111/bcp.13622. Epub 2018 Jun 5. Br J Clin Pharmacol. 2018. PMID: 29708281 Free PMC article. Clinical Trial.
-
OBE022, an Oral and Selective Prostaglandin F2α Receptor Antagonist as an Effective and Safe Modality for the Treatment of Preterm Labor.J Pharmacol Exp Ther. 2018 Aug;366(2):349-364. doi: 10.1124/jpet.118.247668. Epub 2018 May 18. J Pharmacol Exp Ther. 2018. PMID: 29777040
-
Thorough QT study of the effect of oral moxifloxacin on QTc interval in the fed and fasted state in healthy Japanese and Caucasian subjects.Br J Clin Pharmacol. 2014 Jan;77(1):170-9. doi: 10.1111/bcp.12168. Br J Clin Pharmacol. 2014. PMID: 23713767 Free PMC article. Clinical Trial.
-
The IQ-CSRC prospective clinical Phase 1 study: "Can early QT assessment using exposure response analysis replace the thorough QT study?".Ann Noninvasive Electrocardiol. 2014 Jan;19(1):70-81. doi: 10.1111/anec.12128. Epub 2013 Dec 30. Ann Noninvasive Electrocardiol. 2014. PMID: 24372708 Free PMC article. Review.
Cited by
-
Addressing a broken drug pipeline for preterm birth: why early preterm birth is an orphan disease.Am J Obstet Gynecol. 2023 Dec;229(6):647-655. doi: 10.1016/j.ajog.2023.07.042. Epub 2023 Jul 27. Am J Obstet Gynecol. 2023. PMID: 37516401 Free PMC article.
-
Landscape of Preterm Birth Therapeutics and a Path Forward.J Clin Med. 2021 Jun 29;10(13):2912. doi: 10.3390/jcm10132912. J Clin Med. 2021. PMID: 34209869 Free PMC article. Review.
-
A Phase 1 Study to Investigate the Effects of Cortexolone 17α-Propionate, Also Known as Clascoterone, on the QT Interval Using the Meal Effect to Demonstrate ECG Assay Sensitivity.Clin Pharmacol Drug Dev. 2021 Jun;10(6):572-581. doi: 10.1002/cpdd.935. Epub 2021 May 3. Clin Pharmacol Drug Dev. 2021. PMID: 33942574 Free PMC article. Clinical Trial.
-
Coadministration of the prostaglandin F2α receptor antagonist preterm labour drug candidate OBE022 with magnesium sulfate, atosiban, nifedipine and betamethasone.Br J Clin Pharmacol. 2019 Jul;85(7):1516-1527. doi: 10.1111/bcp.13925. Epub 2019 May 11. Br J Clin Pharmacol. 2019. PMID: 30891820 Free PMC article. Clinical Trial.
-
Preterm Birth Therapies to Target Inflammation.J Clin Pharmacol. 2022 Sep;62 Suppl 1(Suppl 1):S79-S93. doi: 10.1002/jcph.2107. J Clin Pharmacol. 2022. PMID: 36106783 Free PMC article.
References
-
- Harrison MS, Goldenberg RL. Global burden of prematurity. Semin Fetal Neonatal Med. 2016;21(2):74–79. - PubMed
-
- van Vliet EOG, Dijkema GH, Schuit E, et al. Nifedipine maintenance tocolysis and perinatal outcome: an individual participant data meta‐analysis. BJOG. 2016;123(11):1753–1760. - PubMed
-
- Jovanović N, Pavlović M, Mirčevski V, Du Q, Jovanović A. An unexpected negative inotropic effect of prostaglandin F2α in the rat heart. Prostaglandins Other Lipid Mediat. 2006;80(1):110–119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

