Sluggish Cognitive Tempo as a Possible Predictor of Methylphenidate Response in Children With ADHD: A Randomized Controlled Trial
- PMID: 29489078
- PMCID: PMC6558969
- DOI: 10.4088/JCP.17m11553
Sluggish Cognitive Tempo as a Possible Predictor of Methylphenidate Response in Children With ADHD: A Randomized Controlled Trial
Abstract
Objective: To examine whether sluggish cognitive tempo (SCT) symptomatology moderates dose response to methylphenidate and whether the impact of SCT on medication response is distinct from attention-deficit/hyperactivity disorder (ADHD) subtype effects.
Methods: Stimulant-naive children with ADHD predominantly inattentive type (ADHD-I; n = 126) or ADHD combined type (ADHD-C; n = 45) aged 7-11 years were recruited from the community from September 2006 to June 2013 to participate in a prospective, randomized, double-blind, 4-week crossover trial of long-acting methylphenidate. ADHD diagnosis and subtype were established according to DSM-IV criteria using a structured interview and teacher ADHD symptom ratings. SCT symptoms were assessed using a teacher-rated scale with 2 factors (Sluggish/Sleepy and Daydreamy). Primary outcomes included (1) categorization of children as methylphenidate responders, methylphenidate nonresponders, or placebo responders by 2 blinded physicians and (2) parent and teacher ratings of child behavior on the Vanderbilt ADHD Diagnostic Rating Scales while subjects were on treatment with placebo or 1 of 3 methylphenidate dosages (low, medium, high).
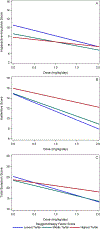
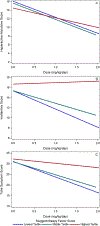
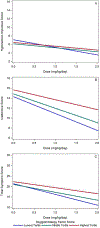
Results: Increased SCT Sluggish/Sleepy factor scores were associated with being a methylphenidate nonresponder or placebo responder rather than a methylphenidate responder (P = .04). Sluggish/Sleepy factor scores were also linked to diminished methylphenidate dose response for parent- and teacher-rated inattention symptoms (Sluggish/Sleepy factor × dose P = .004). SCT Daydreamy symptoms and ADHD subtype (ADHD-I vs ADHD-C) were not associated with methylphenidate responder status and did not moderate methylphenidate dose response for inattention symptoms.
Conclusions: SCT Sluggish/Sleepy symptoms, but not SCT Daydreamy symptoms or ADHD subtype, predicted methylphenidate nonresponse. This novel finding, if replicated, may have important implications for assessing SCT as part of ADHD care.
Trial registration: ClinicalTrials.gov identifier: NCT01727414.
© Copyright 2018 Physicians Postgraduate Press, Inc.
Figures
References
-
- Barkley RA. Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2002;63 Suppl 12:10–15. - PubMed
-
- Safer DJ, Zito JM. Pharmacoepidemiology of Methylphenidate and Other Stimulants for the Treatment of Attention Deficit Hyperactivity Disorder. In: Greenhill L, ed. Ritalin Theory and Practice 2nd ed. Larchmont, NY: Mary Ann Liebert, Inc.; 2000: 7–26.
-
- Becker SP, Marshall SA, McBurnett K. Sluggish cognitive tempo in abnormal child psychology: an historical overview and introduction to the special section. J Abnorm Child Psychol 2014;42(1):1–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

