Global migration of clinical research during the era of trial registration
- PMID: 29489839
- PMCID: PMC5830297
- DOI: 10.1371/journal.pone.0192413
Global migration of clinical research during the era of trial registration
Erratum in
-
Correction: Global migration of clinical research during the era of trial registration.PLoS One. 2018 Jun 26;13(6):e0199952. doi: 10.1371/journal.pone.0199952. eCollection 2018. PLoS One. 2018. PMID: 29944725 Free PMC article.
Abstract
Background: Since the site of human subjects research has public health, regulatory, ethical, economic, and social implications, we sought to determine the global distribution and migration of clinical research using an open-access trial registry.
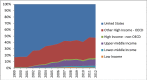
Methods: We obtained individual clinical trial data including location of trial sites, dates of operation, funding source (United States government, pharmaceutical industry, or organization), and clinical study phase (1, 1/2, 2, 2/3, or 3) from ClinicalTrials.gov. We used the World Bank's classification of each country's economic development status ["High Income and a Member of the Organization for Economic Co-operation and Development (OECD)", "High Income and Non-Member of the OECD", "Upper-Middle Income", "Lower-Middle Income", or "Low Income"] and United Nations Populations Division data for country-specific population estimates. We analyzed data from calendar year 2006 through 2012 by number of clinical trial sites, cumulative trial site-years, trial density (trial site-years/106 population), and annual growth rate (%) for each country, and by development category, funding source, and clinical study phase.
Results: Over a 7-year period, 89,647 clinical trials operated 784,585 trial sites in 175 countries, contributing 2,443,850 trial site-years. Among those, 652,200 trial sites (83%) were in 25 high-income OECD countries, while 37,195 sites (5%) were in 91 lower-middle or low-income countries. Trial density (trial site-years/106 population) was 540 in the United States, 202 among other high-income OECD countries (excluding the United States), 81 among high-income non-OECD countries, 41 among upper-middle income countries, 5 among lower-middle income countries, and 2 among low-income countries. Annual compound growth rate was positive (ranging from 0.8% among low-income countries to 14.7% among lower-middle income countries) among all economic groups, except the United States (-0.5%). Overall, 29,191 trials (33%) were funded by industry, 4,059 (5%) were funded by the United States government, and 56,397 (63%) were funded by organizations. Countries with emerging economies (low- and middle-income) operated 19% of phase 3 trial sites, as compared to only 6% of phase 1 trial sites.
Conclusion: Human clinical research remains concentrated in high-income countries, but operational clinical trial sites, particularly for phase 3 trials, may be migrating to low- and middle-income countries with emerging economies.
Conflict of interest statement
Figures




References
-
- Old Testament, Book of Daniel; chapter 1, verses 12–15; describes a planned experiment with both baseline and follow-up observations of two groups who either ate, or did not eat, “the King's meat” over a trial period of ten days.
-
- Anon. Clinical trial of patulin in the common cold. Int J Epidemiol. 33, 243–246 (2004). doi: 10.1093/ije/dyh028 - DOI - PubMed
-
- Munos B. Lessons from 60 years of pharmaceutical innovation. Nature Rev Drug Discov. 8, 959–68 (2009). - PubMed
-
- Thiers F.A., Sinskey A.J., Er B. Trends in the globalization of clinical trials. Nature Rev Drug Discovery. 7, 13–14 (2008).
-
- Brooks, K., Pharma C. CRO Outlook & Opportunities: e-clinical solutions fuel advances. Available at http://www.contractpharma.com/issues/2012-06/view_features/cro-outlook-o.... Accessed on November 10, 2014.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

