Does the rising placebo response impact antihypertensive clinical trial outcomes? An analysis of data from the Food and Drug Administration 1990-2016
- PMID: 29489874
- PMCID: PMC5831097
- DOI: 10.1371/journal.pone.0193043
Does the rising placebo response impact antihypertensive clinical trial outcomes? An analysis of data from the Food and Drug Administration 1990-2016
Abstract
Background: Recent studies show that placebo response has grown significantly over time in clinical trials for antidepressants, ADHD medications, antiepileptics, and antidiabetics. Contrary to expectations, trial outcome measures and success rates have not been impacted. This study aimed to see if this trend of increasing placebo response and stable efficacy outcome measures is unique to the conditions previously studied or if it occurs in trials for conditions with physiologically-measured symptoms, such as hypertension.
Method: For this reason, we evaluated the efficacy data reported in the US Food and Drug Administration Medical and Statistical reviews for 23 antihypertensive programs (32,022 patients, 63 trials, 142 treatment arms). Placebo and medication response, effect sizes, and drug-placebo differences were calculated for each treatment arm and examined over time using meta-regression. We also explored the relationship of sample size, trial duration, baseline blood pressure, and number of treatment arms to placebo/drug response and efficacy outcome measures.
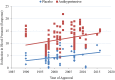
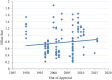
Results: Like trials of other conditions, placebo response has risen significantly over time (R2 = 0.093, p = 0.018) and effect size (R2 = 0.013, p = 0.187) drug-placebo difference (R2 = 0.013, p = 0.182) and success rate (134/142, 94.4%) have remained unaffected, likely due to a significant compensatory increase in antihypertensive response (R2 = 0.086, p<0.001). Treatment arms are likely overpowered with sample sizes increasing over time (R2 = 0.387, p<0.0001) and stable, large effect sizes (0.78 ±0.37). The exploratory analysis of sample size, trial duration, baseline blood pressure, and number of treatment arms yielded mixed results unlikely to explain the pattern of placebo response and efficacy outcomes over time. The magnitude of placebo response had no relationship to effect size (p = 0.877), antihypertensive-placebo differences (p = 0.752), or p-values (p = 0.963) but was correlated with antihypertensive response (R2 = 0.347, p<0.0001).
Conclusions: As hypothesized, this study shows that placebo response is increasing in clinical trials for hypertension without any evidence of this increase impacting trial outcomes. Attempting to control placebo response in clinical trials for hypertension may not be necessary for successful efficacy outcomes. In exploratory analysis, we noted that despite finding significant relationships, none of the trial or patient characteristics we examined offered a clear explanation of the rise in placebo and stability in outcome measures over time. Collectively, these data suggest that the phenomenon of increasing placebo response and stable efficacy outcomes may be a general trend, occurring across trials for various psychiatric and medical conditions with physiological and non-physiological endpoints.
Conflict of interest statement
Figures

References
-
- Andrews G. Placebo response in depression: bane of research, boon to therapy. Br J Psychiatr. 2001;178(3): 192–194. - PubMed
-
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2001;287(14): 1840–1847. - PubMed
-
- Khan A, Detke M, Khan S, Mallinckrodt C. Placebo response and antidepressant clinical trial outcome. J Nerv Ment Dis. 2003;191(4): 211–218. doi: 10.1097/01.NMD.0000061144.16176.38 - DOI - PubMed
-
- Khan A, Fahl Mar K, Faucett J, Khan Schilling S, Brown WA. Has the rising placebo response impacted antidepressant clinical trial outcome? Data from the US Food and Drug Administration 1987–2013. World Psychiatry. 2017;16(2): 181–192. doi: 10.1002/wps.20421 - DOI - PMC - PubMed
-
- Khan A, Fahl Mar K, Brown WA. Does the increasing magnitude of placebo response affect outcome of adult and pediatric ADHD clinical trials? J of Psychiatric Res. 2017;94: 202–207. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

