Significant Patient Impact Observed Upon Implementation of Point-of-Care Early Infant Diagnosis Technologies in an Observational Study in Malawi
- PMID: 29490026
- PMCID: PMC6093992
- DOI: 10.1093/cid/ciy169
Significant Patient Impact Observed Upon Implementation of Point-of-Care Early Infant Diagnosis Technologies in an Observational Study in Malawi
Abstract
Background: In Malawi in 2014, <20% of human immunodeficiency virus (HIV)-exposed infants received an early infant diagnosis (EID) test in the first 2 months of life and only 30% of HIV-infected children were on antiretroviral therapy (ART). We sought to understand the potential patient impact of improving timely infant diagnosis and treatment initiation through implementation of point-of-care (POC) EID technologies in Malawi.
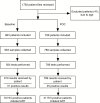
Methods: In this observational study, POC EID technologies were introduced into routine services at 7 health facilities across Malawi in September 2015. The primary outcome was the proportion of HIV-infected infants initiating ART within 60 days of sample collection in the POC arm compared to the baseline arm with conventional laboratory-based EID testing.
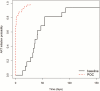
Results: The time from sample collection to result received by the patient decreased significantly from 56 days (interquartile range [IQR], 30-81 days) in the baseline arm to <1 day in the POC arm (P < .001). Of the HIV-infected infants, the time between sample collection and ART initiation was reduced from 38 days (IQR, 30-54 days) in the baseline arm to <1 day (IQR, 0-1 day) in the POC arm (P = .019). Furthermore, the proportion of HIV-infected infants initiated on ART within 60 days of sample collection increased significantly from 41.9% to 91.1% after the introduction of POC (adjusted risk ratio, 2.28; P < .001).
Conclusions: ART initiation rates were significantly improved with the implementation of same-day POC EID testing compared with referred, longer-turnaround laboratory-based testing.
Figures
References
-
- Joint United Nations Programme on HIV/AIDS. AIDS by the numbers. Geneva, Switzerland: UNAIDS, 2016.
-
- Joint United Nations Programme on HIV/AIDS. Children and HIV fact sheet. Geneva, Switzerland: UNAIDS, 2016.
-
- Bourne DE, Thompson M, Brody LL, et al. . Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS 2009; 23:101–6. - PubMed
-
- Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F; Ghent International AIDS Society (IAS) Working Group on HIV Infection in Women and Children Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004; 364:1236–43. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

