Randomized Noninferiority Trial of Telephone vs In-Person Disclosure of Germline Cancer Genetic Test Results
- PMID: 29490071
- PMCID: PMC6136932
- DOI: 10.1093/jnci/djy015
Randomized Noninferiority Trial of Telephone vs In-Person Disclosure of Germline Cancer Genetic Test Results
Abstract
Background: Germline genetic testing is standard practice in oncology. Outcomes of telephone disclosure of a wide range of cancer genetic test results, including multigene panel testing (MGPT) are unknown.
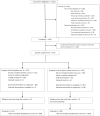
Methods: Patients undergoing cancer genetic testing were recruited to a multicenter, randomized, noninferiority trial (NCT01736345) comparing telephone disclosure (TD) of genetic test results with usual care, in-person disclosure (IPD) after tiered-binned in-person pretest counseling. Primary noninferiority outcomes included change in knowledge, state anxiety, and general anxiety. Secondary outcomes included cancer-specific distress, depression, uncertainty, satisfaction, and screening and risk-reducing surgery intentions. To declare noninferiority, we calculated the 98.3% one-sided confidence interval of the standardized effect; t tests were used for secondary subgroup analyses. Only noninferiority tests were one-sided, others were two-sided.
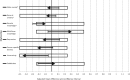
Results: A total of 1178 patients enrolled in the study. Two hundred eight (17.7%) participants declined random assignment due to a preference for in-person disclosure; 473 participants were randomly assigned to TD and 497 to IPD; 291 (30.0%) had MGPT. TD was noninferior to IPD for general and state anxiety and all secondary outcomes immediately postdisclosure. TD did not meet the noninferiority threshold for knowledge in the primary analysis, but it did meet the threshold in the multiple imputation analysis. In secondary analyses, there were no statistically significant differences between arms in screening and risk-reducing surgery intentions, and no statistically significant differences in outcomes by arm among those who had MGPT. In subgroup analyses, patients with a positive result had statistically significantly greater decreases in general anxiety with telephone disclosure (TD -0.37 vs IPD +0.87, P = .02).
Conclusions: Even in the era of multigene panel testing, these data suggest that telephone disclosure of cancer genetic test results is as an alternative to in-person disclosure for interested patients after in-person pretest counseling with a genetic counselor.
Figures
Similar articles
-
Preferences for in-person disclosure: Patients declining telephone disclosure characteristics and outcomes in the multicenter Communication Of GENetic Test Results by Telephone study.Clin Genet. 2019 Feb;95(2):293-301. doi: 10.1111/cge.13474. Epub 2018 Dec 7. Clin Genet. 2019. PMID: 30417332 Free PMC article. Clinical Trial.
-
Longitudinal follow-up after telephone disclosure in the randomized COGENT study.Genet Med. 2020 Aug;22(8):1401-1406. doi: 10.1038/s41436-020-0808-3. Epub 2020 May 7. Genet Med. 2020. PMID: 32376981 Free PMC article. Clinical Trial.
-
Implementation and outcomes of telephone disclosure of clinical BRCA1/2 test results.Patient Educ Couns. 2013 Dec;93(3):413-9. doi: 10.1016/j.pec.2013.08.009. Epub 2013 Aug 19. Patient Educ Couns. 2013. PMID: 24075727 Free PMC article.
-
The Disclosure of Bad News Over the Phone vs. in Person and its Association with Psychological Distress: a Systematic Review and Meta-Analysis.J Gen Intern Med. 2023 Dec;38(16):3589-3603. doi: 10.1007/s11606-023-08323-z. Epub 2023 Aug 8. J Gen Intern Med. 2023. PMID: 37552418 Free PMC article.
-
Comparing Outcomes of Genetic Counseling Options in Breast and Ovarian Cancer: An Integrative Review .Oncol Nurs Forum. 2018 Jan 1;45(1):96-105. doi: 10.1188/18.ONF.96-105. Oncol Nurs Forum. 2018. PMID: 29251290 Review.
Cited by
-
Uptake of Genetic Research Results and Patient-Reported Outcomes With Return of Results Incorporating Web-Based Predisclosure Education.J Clin Oncol. 2023 Nov 1;41(31):4905-4915. doi: 10.1200/JCO.22.00516. Epub 2023 Aug 23. J Clin Oncol. 2023. PMID: 37611220 Free PMC article.
-
Cascade Genetic Testing of Relatives for Hereditary Cancer Risk: Results of an Online Initiative.J Natl Cancer Inst. 2019 Jan 1;111(1):95-98. doi: 10.1093/jnci/djy147. J Natl Cancer Inst. 2019. PMID: 30239769 Free PMC article.
-
Transitioning to telegenetics in the COVID-19 era: Patient satisfaction with remote genetic counseling in adult neurology.J Genet Couns. 2021 Aug;30(4):974-983. doi: 10.1002/jgc4.1470. Epub 2021 Jul 15. J Genet Couns. 2021. PMID: 34265143 Free PMC article.
-
Improved Access to Genetics Care is Needed to Address Health Inequities in ATTRv Amyloidosis.Circulation. 2024 Oct;150(14):1072-1074. doi: 10.1161/CIRCULATIONAHA.124.070525. Epub 2024 Sep 30. Circulation. 2024. PMID: 39348455
-
Longitudinal outcomes with cancer multigene panel testing in previously tested BRCA1/2 negative patients.Clin Genet. 2020 Apr;97(4):601-609. doi: 10.1111/cge.13716. Clin Genet. 2020. PMID: 32022897 Free PMC article.
References
-
- Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;12424:2761–2796.10.1161/CIR.0b013e318223e230 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

