PHLDA1 Mediates Drug Resistance in Receptor Tyrosine Kinase-Driven Cancer
- PMID: 29490281
- PMCID: PMC5848852
- DOI: 10.1016/j.celrep.2018.02.028
PHLDA1 Mediates Drug Resistance in Receptor Tyrosine Kinase-Driven Cancer
Abstract
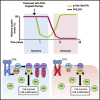
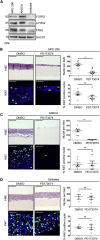
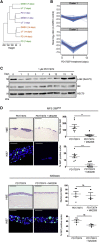
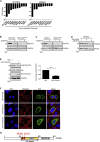
Development of resistance causes failure of drugs targeting receptor tyrosine kinase (RTK) networks and represents a critical challenge for precision medicine. Here, we show that PHLDA1 downregulation is critical to acquisition and maintenance of drug resistance in RTK-driven cancer. Using fibroblast growth factor receptor (FGFR) inhibition in endometrial cancer cells, we identify an Akt-driven compensatory mechanism underpinned by downregulation of PHLDA1. We demonstrate broad clinical relevance of our findings, showing that PHLDA1 downregulation also occurs in response to RTK-targeted therapy in breast and renal cancer patients, as well as following trastuzumab treatment in HER2+ breast cancer cells. Crucially, knockdown of PHLDA1 alone was sufficient to confer de novo resistance to RTK inhibitors and induction of PHLDA1 expression re-sensitized drug-resistant cancer cells to targeted therapies, identifying PHLDA1 as a biomarker for drug response and highlighting the potential of PHLDA1 reactivation as a means of circumventing drug resistance.
Keywords: Akt; FGF; cancer; drug resistance; targeted therapy; tyrosine kinase inhibitor.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
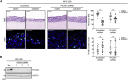

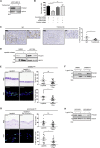

References
-
- Byron S.A., Gartside M.G., Wellens C.L., Mallon M.A., Keenan J.B., Powell M.A., Goodfellow P.J., Pollock P.M. Inhibition of activated fibroblast growth factor receptor 2 in endometrial cancer cells induces cell death despite PTEN abrogation. Cancer Res. 2008;68:6902–6907. - PubMed
-
- Byron S.A., Loch D.C., Pollock P.M. Fibroblast growth factor receptor inhibition synergizes with Paclitaxel and Doxorubicin in endometrial cancer cells. Int. J. Gynecol. Cancer. 2012;22:1517–1526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

