QTL mapping of volatile compound production in Saccharomyces cerevisiae during alcoholic fermentation
- PMID: 29490607
- PMCID: PMC5831830
- DOI: 10.1186/s12864-018-4562-8
QTL mapping of volatile compound production in Saccharomyces cerevisiae during alcoholic fermentation
Abstract
Background: The volatile metabolites produced by Saccharomyces cerevisiae during alcoholic fermentation, which are mainly esters, higher alcohols and organic acids, play a vital role in the quality and perception of fermented beverages, such as wine. Although the metabolic pathways and genes behind yeast fermentative aroma formation are well described, little is known about the genetic mechanisms underlying variations between strains in the production of these aroma compounds. To increase our knowledge about the links between genetic variation and volatile production, we performed quantitative trait locus (QTL) mapping using 130 F2-meiotic segregants from two S. cerevisiae wine strains. The segregants were individually genotyped by next-generation sequencing and separately phenotyped during wine fermentation.
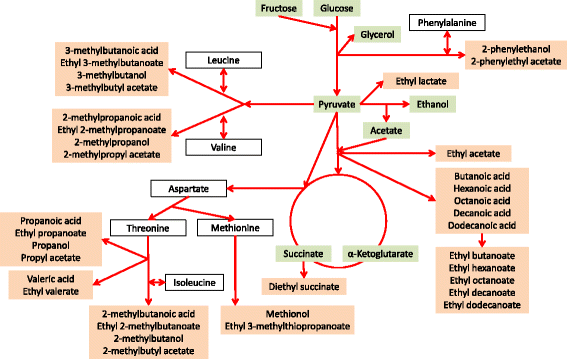
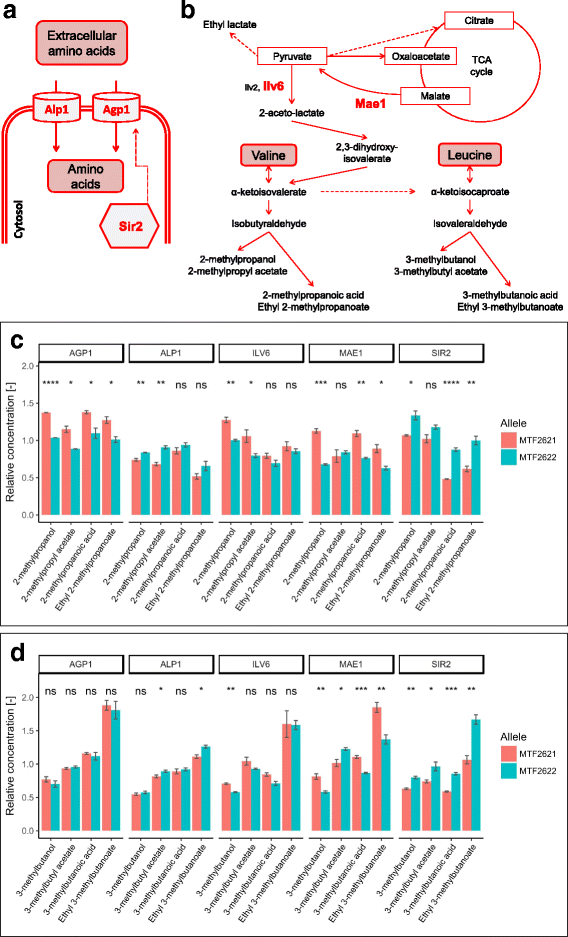
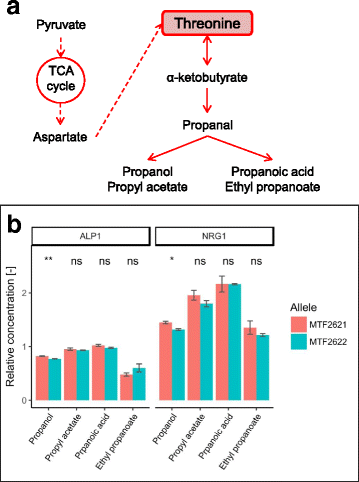
Results: Using different QTL mapping strategies, we were able to identify 65 QTLs in the genome, including 55 that influence the formation of 30 volatile secondary metabolites, 14 with an effect on sugar consumption and central carbon metabolite production, and 7 influencing fermentation parameters. For ethyl lactate, ethyl octanoate and propanol formation, we discovered 2 interacting QTLs each. Within 9 of the detected regions, we validated the contribution of 13 genes in the observed phenotypic variation by reciprocal hemizygosity analysis. These genes are involved in nitrogen uptake and metabolism (AGP1, ALP1, ILV6, LEU9), central carbon metabolism (HXT3, MAE1), fatty acid synthesis (FAS1) and regulation (AGP2, IXR1, NRG1, RGS2, RGT1, SIR2) and explain variations in the production of characteristic sensorial esters (e.g., 2-phenylethyl acetate, 2-metyhlpropyl acetate and ethyl hexanoate), higher alcohols and fatty acids.
Conclusions: The detection of QTLs and their interactions emphasizes the complexity of yeast fermentative aroma formation. The validation of underlying allelic variants increases knowledge about genetic variation impacting metabolic pathways that lead to the synthesis of sensorial important compounds. As a result, this work lays the foundation for tailoring S. cerevisiae strains with optimized volatile metabolite production for fermented beverages and other biotechnological applications.
Keywords: Aroma compounds; Fermentation; Metabolites; QTL mapping; Yeast.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Lambrechts MG, Pretorius IS. Yeast and its importance to wine aroma - a review. South African J Enol Vitic. 2000;21:97–129.
-
- Nykänen L, Suomalainen H. Aroma of beer, wine and distilled alcoholic beverages. Berlin: Springer Science & Business Media; 1983.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

