Effect of an vitamin D deficiency on depressive symptoms in child and adolescent psychiatric patients - a randomized controlled trial: study protocol
- PMID: 29490621
- PMCID: PMC5831612
- DOI: 10.1186/s12888-018-1637-7
Effect of an vitamin D deficiency on depressive symptoms in child and adolescent psychiatric patients - a randomized controlled trial: study protocol
Abstract
Background: Depression is a significant health and economic burden worldwide affecting not only adults but also children and adolescents. Current treatment options for this group are scarce and show moderate effect sizes. There is emerging evidence that dietary patterns and specific nutritional components might play a role in the risk for developing depression. This study protocol focusses on the role of vitamin D which is for long known to be relevant for calcium and phosphorous homeostasis and bone health but might also impact on mental health. However, the assessment of the vitamin D status of depressed juvenile patients, or supplementation of vitamin D is currently not part of routine treatment. Controlled intervention studies are indispensable to prove whether a vitamin D deficiency ameliorates depressive symptoms.
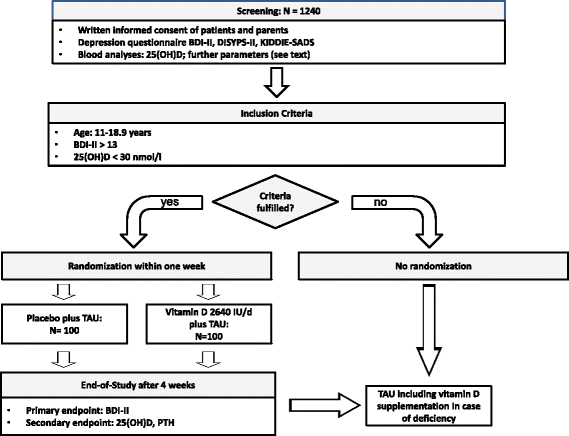
Methods/design: This double blinded, randomized controlled trial will enroll 200 inpatients from a child and adolescent psychiatric department with a vitamin D deficiency defined by a 25(OH)-vitamin D-level < 30 nmol/l (12 ng/ml) and a Beck Depressions Inventory (BDI-II) score > 13 (indicating at least: mild depression). Upon referral, all patients will be screened, checked for inclusion criteria, and those eligible will be randomized after written consent into a supplementation or placebo group. Both study-arms will receive treatment-as-usual for their psychiatric disorder according to established clinical guidelines. The participants of the vitamin D supplementation group will receive 2640 I.E. vitamin D3 q.d. for 28 days in accordance with best practice in pediatric endocrinology. We hypothesize that delaying supplementation of vitamin D in the placebo arm will affect the treatment success of the depressive symptomatology in comparison to the vitamin D supplementation group. Patients will be enrolled for a period of 28 days based on the mean length of hospitalization of juveniles with depression.
Discussion: Randomized controlled trials in children and adolescents with depression are needed to elucidate the role of a vitamin D deficiency for mental disorders and to investigate the relevance of a routine assessment and supplementation of vitamin D deficits.
Trial registration: DRKS00009758, 16/06/2016 (retrospectively registered).
Keywords: Children & Adolescents; Depression; Mental health; Vitamin D.
Conflict of interest statement
Ethics approval and consent to participate
The study was designed and is being conducted according to the latest version of the Declaration of Helsinki [81] and Good Clinical Practice (ICH-GCP (E6)) [82]. The Institutional Review Board (“Ethik-Kommission der Medizinischen Fakultät der Universität Duisburg-Essen”) of the University Duisburg- Essen gave ethical approval (project approval number: 15–6363-BO) to study design, content and recruitment plans. Written informed consent is being obtained from all enrolled participants and their parents (in case participant is younger than eighteen years) after they had the opportunity to ask all open questions and received a satisfying answer from the study researcher.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest. Dr. B. Scheffler Nachf. GmbH & Co.KG, Bergisch Gladbach, Germany, confirms that it exerts no influence on the results and the evaluation and assessment of the study “Effect of an vitamin D deficiency on depressive symptoms in child and adolescent psychiatric patients – a randomized controlled trial”.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Jobst A, Brakemeier EL, Buchheim A, Caspar F, Cuijpers P, Ebmeier KP, Falkai P, Jan van der Gaag R, Gaebel W, Herpertz S, et al. European psychiatric association guidance on psychotherapy in chronic depression across Europe. Eur Psychiatry. 2016;33:18–36. doi: 10.1016/j.eurpsy.2015.12.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

