Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: a population-based study
- PMID: 29490625
- PMCID: PMC5831576
- DOI: 10.1186/s12885-018-4138-7
Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: a population-based study
Abstract
Background: There is no consensus regarding the optimal time to initiate adjuvant chemotherapy after surgery for stage III colon cancer, and the relevant postoperative complications that cause delays in adjuvant chemotherapy are unknown.
Methods: Eligible patients aged ≥66 years who were diagnosed with stage III colon cancer from 1992 to 2008 were identified using the linked Surveillance, Epidemiology, and End Results-Medicare database. Kaplan-Meier analysis and a Cox proportional hazards model were utilized to evaluate the impact of the timing of adjuvant chemotherapy on overall survival (OS).
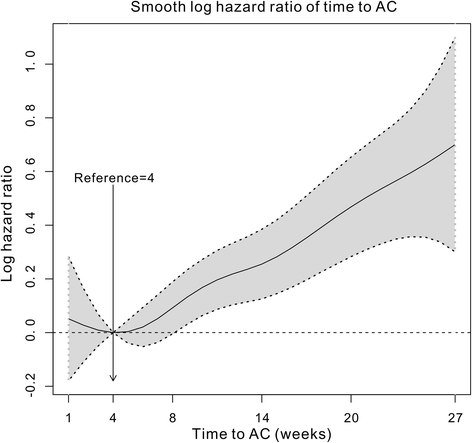
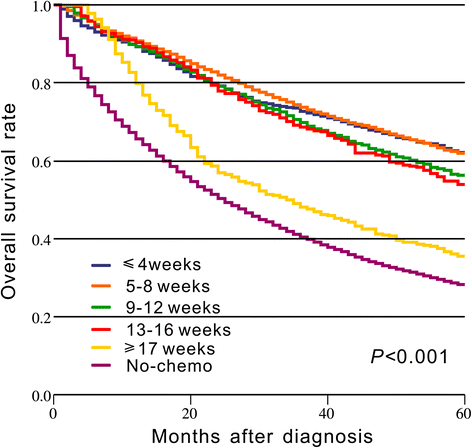
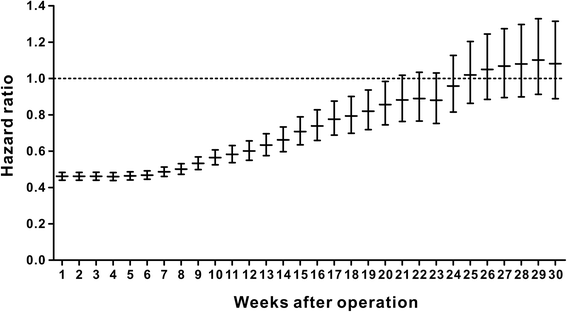
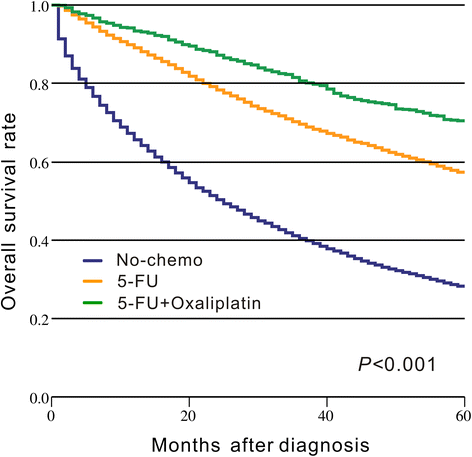
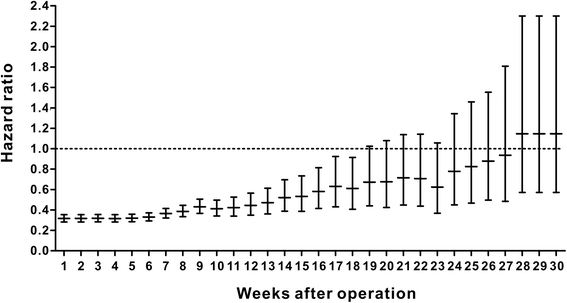
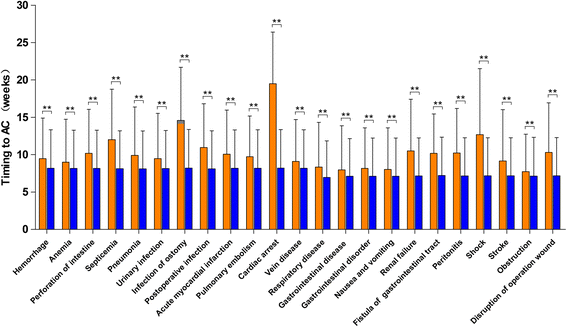
Results: A total of 18,491 patients were included. Delayed adjuvant chemotherapy was associated with worse OS (9-12 weeks: hazard ratio [HR] = 1.222, 95% confidence interval [CI] = 1.063-1.405; 13-16 weeks: HR = 1.252, 95% CI = 1.041-1.505; ≥ 17 weeks: HR = 1.969, 95% CI = 1.663-2.331). The efficacies of adjuvant chemotherapy within 5-8 weeks and ≤4 weeks were similar (HR = 1.045, 95% CI = 0.921-1.185). Compared with the non-chemotherapy group, chemotherapy initiated at ≥21 weeks did not significantly improve OS (HR = 0.882, 95% CI = 0.763-1.018). Patients with postoperative complications, particularly cardiac arrest, ostomy infection, shock, and septicemia, had a significantly higher risk of a 4- to 11-week delay in adjuvant chemotherapy (p < 0.05).
Conclusions: Adjuvant chemotherapy initiated within 8 weeks was acceptable for patients with stage III colon cancer. Delayed adjuvant chemotherapy after 8 weeks was significantly associated with worse OS. However, adjuvant chemotherapy might still be useful even with a delay of approximately 5 months. Moreover, postoperative complications were significantly associated with delayed adjuvant chemotherapy.
Keywords: Colon cancer; Postoperative complications; SEER-Medicare program; Stage III; Timing of adjuvant chemotherapy.
Conflict of interest statement
Ethics approval and consent to participate
Because the SEER-Medicare data are de-identified and are based on registry data, no prior informed consent was required. The access to the SEER-Medicare database was approved by the National Cancer Institute and Information Management Services, Inc. (D6-MEDIC-821), while this study was approved by the Institutional Review Board of the First Hospital of China Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer.Eur J Cancer. 2015 Nov;51(17):2553-61. doi: 10.1016/j.ejca.2015.08.016. Epub 2015 Sep 7. Eur J Cancer. 2015. PMID: 26360411
-
Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer.Cancer. 2006 Dec 1;107(11):2581-8. doi: 10.1002/cncr.22316. Cancer. 2006. PMID: 17078055
-
Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer.Cancer. 2015 Feb 15;121(4):527-34. doi: 10.1002/cncr.29072. Epub 2014 Oct 20. Cancer. 2015. PMID: 25332117
-
Assessing the Effect of Radiotherapy in Addition to Surgery in Colon Adenocarcinomas: a Systematic Review and Meta-analysis of Contemporary Evidence.J Gastrointest Cancer. 2020 Jun;51(2):445-460. doi: 10.1007/s12029-019-00300-2. J Gastrointest Cancer. 2020. PMID: 31463890
-
Timing of Adjuvant Chemotherapy and Survival in Colorectal, Gastric, and Pancreatic Cancer. A Systematic Review and Meta-Analysis.Cancers (Basel). 2019 Apr 17;11(4):550. doi: 10.3390/cancers11040550. Cancers (Basel). 2019. PMID: 30999653 Free PMC article. Review.
Cited by
-
Utilization of tissue-free minimal residual disease testing in colorectal cancer patients from Asia and Middle East.Front Oncol. 2024 Sep 20;14:1426941. doi: 10.3389/fonc.2024.1426941. eCollection 2024. Front Oncol. 2024. PMID: 39372864 Free PMC article.
-
Disparities in Characteristics, Access to Care, and Oncologic Outcomes in Young-Onset Colorectal Cancer at a Safety-Net Hospital.JCO Oncol Pract. 2021 May;17(5):e614-e622. doi: 10.1200/OP.20.00777. Epub 2021 Jan 11. JCO Oncol Pract. 2021. PMID: 33428470 Free PMC article.
-
TreatmENT of AnastomotiC LeakagE after colon cancer resection: the TENTACLE - Colon study.BMC Surg. 2025 May 15;25(1):213. doi: 10.1186/s12893-025-02954-1. BMC Surg. 2025. PMID: 40375249 Free PMC article.
-
Adjuvant Chemotherapy for Stage III Colon Cancer.Cancers (Basel). 2020 Sep 19;12(9):2679. doi: 10.3390/cancers12092679. Cancers (Basel). 2020. PMID: 32961795 Free PMC article. Review.
-
The importance of time-to-adjuvant treatment on survival with pancreatic cancer: A systematic review and meta-analysis.Cancer Rep (Hoboken). 2021 Oct;4(5):e1390. doi: 10.1002/cnr2.1390. Epub 2021 Jul 10. Cancer Rep (Hoboken). 2021. PMID: 34245139 Free PMC article.
References
-
- Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, Haller DG, Benedetti J, Francini G, Shepherd LE, Francois Seitz J, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(10):1797–1806. doi: 10.1200/JCO.2004.09.059. - DOI - PubMed
-
- Fisher B, Gunduz N, Coyle J, Rudock C, Saffer E. Presence of a growth-stimulating factor in serum following primary tumor removal in mice. Cancer Res. 1989;49(8):1996–2001. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

