Effect of an exercise-based cardiac rehabilitation program "Baduanjin Eight-Silken-Movements with self-efficacy building" for heart failure (BESMILE-HF study): study protocol for a randomized controlled trial
- PMID: 29490680
- PMCID: PMC5831846
- DOI: 10.1186/s13063-018-2531-9
Effect of an exercise-based cardiac rehabilitation program "Baduanjin Eight-Silken-Movements with self-efficacy building" for heart failure (BESMILE-HF study): study protocol for a randomized controlled trial
Abstract
Background: Exercise-based cardiac rehabilitation is a beneficial therapy for patients with chronic heart failure. The delivery of exercise-based cardiac rehabilitation should adopt an evidence-based approach, as well as be culturally appropriate and sensitive to individual needs and preferences. The Baduanjin Eight-Silken-Movements with Self-efficacy Building for Heart Failure (BESMILE-HF) program is the first to apply a traditional Chinese exercise, Baduanjin, as the core component in an exercise-based cardiac rehabilitation program. This trial aims to assess the efficacy, safety, and acceptability of the addition of the BESMILE-HF program to usual medications for patients with chronic heart failure.
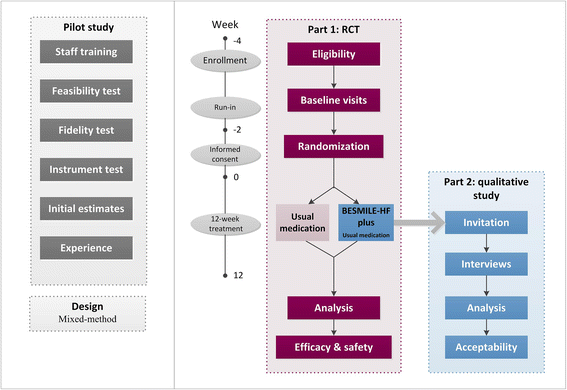
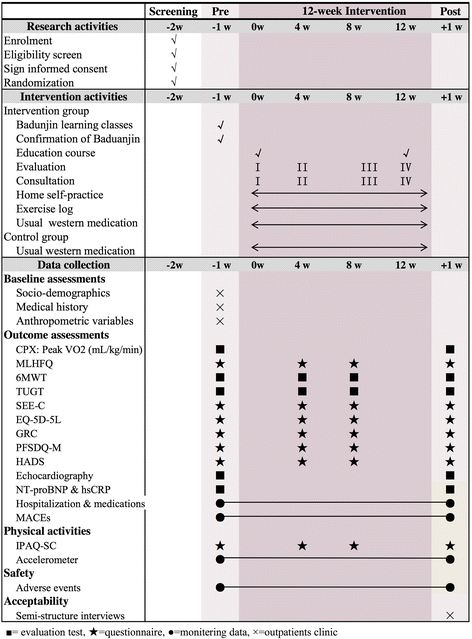
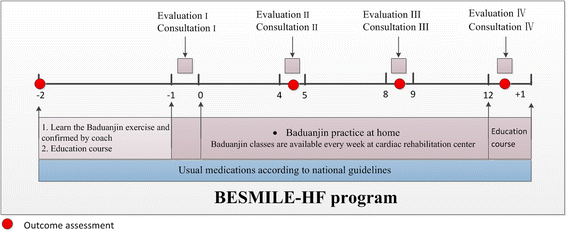
Methods/design: The BESMILE-HF study is a mixed-design study. It includes a two-group, parallel, randomized controlled trial with 200 chronic heart failure patients, as well as a qualitative component. Patients will be randomized into either an intervention group receiving the 12-week BESMILE-HF program plus usual medications, or a control group receiving only usual medications. The primary outcomes are peak oxygen consumption assessed using a cardiopulmonary exercise test, and disease-specific quality of life using the Minnesota Living with Heart Failure Questionnaire. The secondary outcomes are: exercise performance, exercise self-efficacy, general quality of life, dyspnea and fatigue, depression, cardiac function, prognostic and inflammatory indicator levels, hospitalization, use of medications, and major adverse cardiac events. Assessments will be carried out at baseline, and at the 4th week, 8th week, and 12th week. The qualitative component will include a semi-structure interview describing patients' experiences with the intervention.
Discussion: This study can provide evidence for how to deliver a contextually adapted exercise-based cardiac rehabilitation program with the potential to be scaled up throughout China.
Trial registration: ClinicalTrials.gov, ID: NCT03180320 . Registered on 2 June 2017.
Keywords: Baduanjin; Chronic heart failure; Exercise capacity; Exercise-based cardiac rehabilitation; Quality of life.
Conflict of interest statement
Ethics approval and consent to participate
The BESMILE-HF study has been approved by the Ethics Committee of the GPHCM (number: B2016–202-01). They will review the study progress at least once a year. A formal amendment to the protocol will be required, and must be approved by the Ethics Committee of the GPHCM prior to implementation, if modifications may impact on the conduct of the study, potential benefit of the patient or may affect patient safety. The BESMILE-HF study will be conducted according to the principles of the Declaration of Helsinki (2013 version). For the RCT, trained research nurses will introduce and discuss the trial information to interested patients, and a signed informed consent form will be obtained before each participant’s enrollment. Participants will be free to withdraw at any time. For the qualitative study, patients within the RCT will be recruited, and interviews and recordings will be conducted after written permission has been given.
Consent for publication
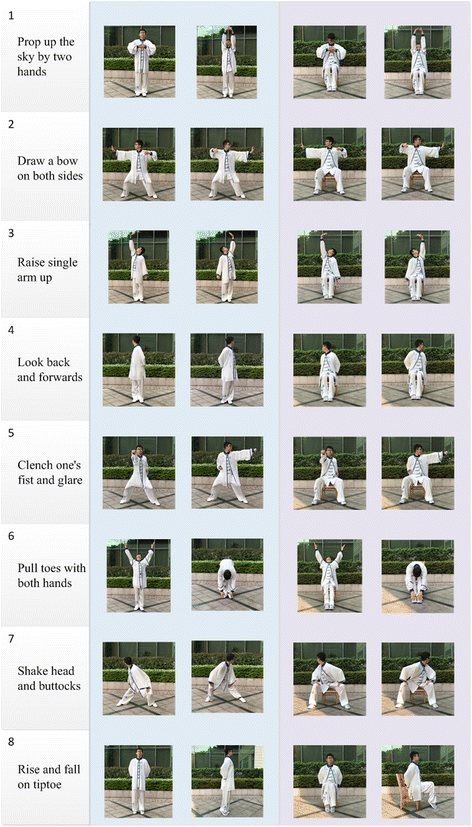
The participant in Fig. 1 has agreed and consented for Fig. 1 to be published.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, et al. Heart Disease and Stroke Statistics – 2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. - DOI - PubMed
-
- 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2016;22(9):659–669. doi: 10.1016/j.cardfail.2016.07.001. - DOI - PubMed
-
- Fleg JL, Cooper LS, Borlaug BA, Haykowsky MJ, Kraus WE, Levine BD, Pfeffer MA, Pina IL, Poole DC, Reeves GR, et al. Exercise training as therapy for heart failure: current status and future directions. Circ Heart Fail. 2015;8(1):209–220. doi: 10.1161/CIRCHEARTFAILURE.113.001420. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

