"Candidatus Paraporphyromonas polyenzymogenes" encodes multi-modular cellulases linked to the type IX secretion system
- PMID: 29490697
- PMCID: PMC5831590
- DOI: 10.1186/s40168-018-0421-8
"Candidatus Paraporphyromonas polyenzymogenes" encodes multi-modular cellulases linked to the type IX secretion system
Abstract
Background: In nature, obligate herbivorous ruminants have a close symbiotic relationship with their gastrointestinal microbiome, which proficiently deconstructs plant biomass. Despite decades of research, lignocellulose degradation in the rumen has thus far been attributed to a limited number of culturable microorganisms. Here, we combine meta-omics and enzymology to identify and describe a novel Bacteroidetes family ("Candidatus MH11") composed entirely of uncultivated strains that are predominant in ruminants and only distantly related to previously characterized taxa.
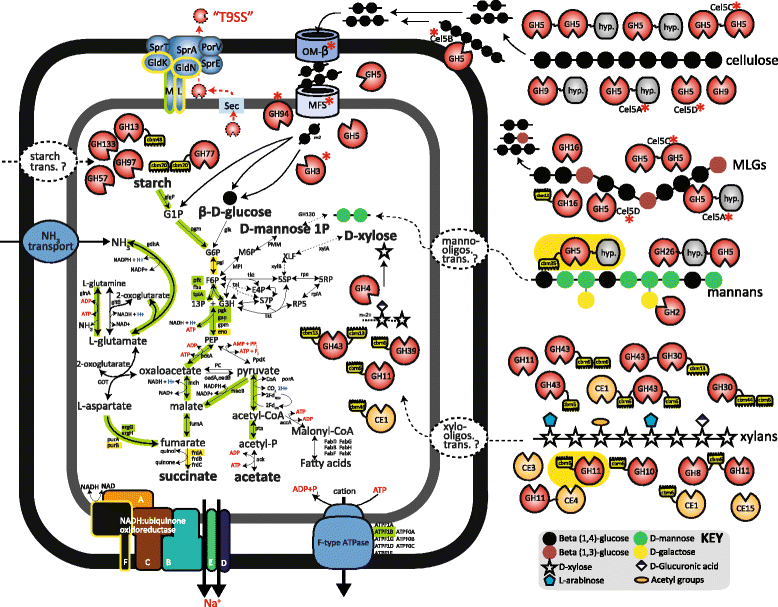
Results: The first metabolic reconstruction of Ca. MH11-affiliated genome bins, with a particular focus on the provisionally named "Candidatus Paraporphyromonas polyenzymogenes", illustrated their capacity to degrade various lignocellulosic substrates via comprehensive inventories of singular and multi-modular carbohydrate active enzymes (CAZymes). Closer examination revealed an absence of archetypical polysaccharide utilization loci found in human gut microbiota. Instead, we identified many multi-modular CAZymes putatively secreted via the Bacteroidetes-specific type IX secretion system (T9SS). This included cellulases with two or more catalytic domains, which are modular arrangements that are unique to Bacteroidetes species studied to date. Core metabolic proteins from Ca. P. polyenzymogenes were detected in metaproteomic data and were enriched in rumen-incubated plant biomass, indicating that active saccharification and fermentation of complex carbohydrates could be assigned to members of this novel family. Biochemical analysis of selected Ca. P. polyenzymogenes CAZymes further iterated the cellulolytic activity of this hitherto uncultured bacterium towards linear polymers, such as amorphous and crystalline cellulose as well as mixed linkage β-glucans.
Conclusion: We propose that Ca. P. polyenzymogene genotypes and other Ca. MH11 members actively degrade plant biomass in the rumen of cows, sheep and most likely other ruminants, utilizing singular and multi-domain catalytic CAZymes secreted through the T9SS. The discovery of a prominent role of multi-modular cellulases in the Gram-negative Bacteroidetes, together with similar findings for Gram-positive cellulosomal bacteria (Ruminococcus flavefaciens) and anaerobic fungi (Orpinomyces sp.), suggests that complex enzymes are essential and have evolved within all major cellulolytic dominions inherent to the rumen.
Keywords: Bacteroidetes; Carbohydrate-active enzymes; Cellulases; Type IX secretion system.
Conflict of interest statement
Ethics approval and consent to participate
All animal procedures were carried out under an animal care and use protocol (IUCAC #06081) approved by the Committee for Animal Care and Use of Animals at the University of Illinois.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

