PER2 regulation of mammary gland development
- PMID: 29490985
- PMCID: PMC5897596
- DOI: 10.1242/dev.157966
PER2 regulation of mammary gland development
Abstract
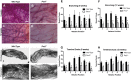
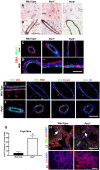
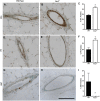
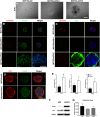
The molecular clock plays key roles in daily physiological functions, development and cancer. Period 2 (PER2) is a repressive element, which inhibits transcription activated by positive clock elements, resulting in diurnal cycling of genes. However, there are gaps in our understanding of the role of the clock in normal development outside of its time-keeping function. Here, we show that PER2 has a noncircadian function that is crucial to mammalian mammary gland development. Virgin Per2-deficient mice, Per2-/- , have underdeveloped glands, containing fewer bifurcations and terminal ducts than glands of wild-type mice. Using a transplantation model, we show that these changes are intrinsic to the gland and further identify changes in cell fate commitment. Per2-/- mouse mammary glands have a dual luminal/basal phenotypic character in cells of the ductal epithelium. We identified colocalization of E-cadherin and keratin 14 in luminal cells. Similar results were demonstrated using MCF10A and shPER2 MCF10A human cell lines. Collectively this study reveals a crucial noncircadian function of PER2 in mammalian mammary gland development, validates the Per2-/- model, and describes a potential role for PER2 in breast cancer.
Keywords: Mammary gland development; Molecular clock; PER2.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
References
-
- Asselin-Labat M.-L., Sutherland K. D., Barker H., Thomas R., Shackleton M., Forrest N. C., Hartley L., Robb L., Grosveld F. G., van der Wees J. et al. (2007). Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat. Cell Biol. 9, 201-209. 10.1038/ncb1530 - DOI - PubMed
-
- Bolos V., Peinado H., Pérez-Moreno M. A., Fraga M. F., Esteller M. and Cano A. (2003). The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116, 499-511. 10.1242/jcs.00224 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

