Vascular Smooth Muscle-Specific Progerin Expression Accelerates Atherosclerosis and Death in a Mouse Model of Hutchinson-Gilford Progeria Syndrome
- PMID: 29490993
- PMCID: PMC6075893
- DOI: 10.1161/CIRCULATIONAHA.117.030856
Vascular Smooth Muscle-Specific Progerin Expression Accelerates Atherosclerosis and Death in a Mouse Model of Hutchinson-Gilford Progeria Syndrome
Abstract
Background: Progerin, an aberrant protein that accumulates with age, causes the rare genetic disease Hutchinson-Gilford progeria syndrome (HGPS). Patients who have HGPS exhibit ubiquitous progerin expression, accelerated aging and atherosclerosis, and die in their early teens, mainly of myocardial infarction or stroke. The mechanisms underlying progerin-induced atherosclerosis remain unexplored, in part, because of the lack of appropriate animal models.
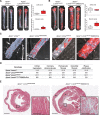
Methods: We generated an atherosclerosis-prone model of HGPS by crossing apolipoprotein E-deficient (Apoe-/-) mice with LmnaG609G/G609G mice ubiquitously expressing progerin. To induce progerin expression specifically in macrophages or vascular smooth muscle cells (VSMCs), we crossed Apoe-/-LmnaLCS/LCS mice with LysMCre and SM22αCre mice, respectively. Progerin expression was evaluated by polymerase chain reaction and immunofluorescence. Cardiovascular alterations were determined by immunofluorescence and histology in male mice fed normal chow or a high-fat diet. In vivo low-density lipoprotein retention was assessed by intravenous injection of fluorescently labeled human low-density lipoprotein. Cardiac electric defects were evaluated by electrocardiography.
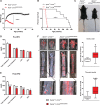
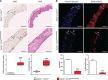
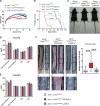
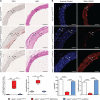
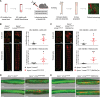
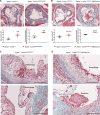
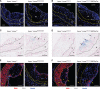
Results: Apoe-/-LmnaG609G/G609G mice with ubiquitous progerin expression exhibited a premature aging phenotype that included failure to thrive and shortened survival. In addition, high-fat diet-fed Apoe-/-LmnaG609G/G609G mice developed a severe vascular pathology, including medial VSMC loss and lipid retention, adventitial fibrosis, and accelerated atherosclerosis, thus resembling most aspects of cardiovascular disease observed in patients with HGPS. The same vascular alterations were also observed in Apoe-/-LmnaLCS/LCSSM22αCre mice expressing progerin specifically in VSMCs, but not in Apoe-/-LmnaLCS/LCSLysMCre mice with macrophage-specific progerin expression. Moreover, Apoe-/-LmnaLCS/LCSSM22αCre mice had a shortened lifespan despite the lack of any overt aging phenotype. Aortas of ubiquitously and VSMC-specific progerin-expressing mice exhibited increased retention of fluorescently labeled human low-density lipoprotein, and atheromata in both models showed vulnerable plaque features. Immunohistopathological examination indicated that Apoe-/-LmnaLCS/LCSSM22αCre mice, unlike Apoe-/-LmnaG609G/G609G mice, die of atherosclerosis-related causes.
Conclusions: We have generated the first mouse model of progerin-induced atherosclerosis acceleration, and demonstrate that restricting progerin expression to VSMCs is sufficient to accelerate atherosclerosis, trigger plaque vulnerability, and reduce lifespan. Our results identify progerin-induced VSMC death as a major factor triggering atherosclerosis and premature death in HGPS.
Keywords: aging; atherosclerosis; cardiovascular diseases; models, animal; muscle, smooth, vascular; progeria.
© 2018 The Authors.
Figures
Comment in
-
Permanently Farnesylated Prelamin A, Progeria, and Atherosclerosis.Circulation. 2018 Jul 17;138(3):283-286. doi: 10.1161/CIRCULATIONAHA.118.034480. Circulation. 2018. PMID: 30012702 Free PMC article. No abstract available.
-
Accelerated atherosclerosis in HGPS.Aging (Albany NY). 2018 Oct 21;10(10):2555-2556. doi: 10.18632/aging.101608. Aging (Albany NY). 2018. PMID: 30348906 Free PMC article. No abstract available.
References
-
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. - PubMed
-
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. - PMC - PubMed
-
- De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, Lévy N. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. - PubMed
-
- Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. - PMC - PubMed
-
- Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A. 2006;140:2603–2624. doi: 10.1002/ajmg.a.31346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

