Disulfide isomerase activity of the dynamic, trimeric Proteus mirabilis ScsC protein is primed by the tandem immunoglobulin-fold domain of ScsB
- PMID: 29491145
- PMCID: PMC5912455
- DOI: 10.1074/jbc.RA118.001860
Disulfide isomerase activity of the dynamic, trimeric Proteus mirabilis ScsC protein is primed by the tandem immunoglobulin-fold domain of ScsB
Abstract
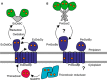
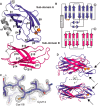
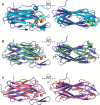
Correct disulfide bond formation is essential for proper folding of many proteins, including bacterial virulence factors. The suppressor of copper sensitivity (Scs) proteins have roles in dithiol/disulfide interchange and the bacterial response to copper stress. Encoded in a four-gene cassette (ScsABCD) present in many Gram-negative bacteria, the Scs proteins are enigmatic and poorly characterized. Here, we show that the periplasmic α-domain of the membrane protein ScsB in the Gram-negative bacterium Proteus mirabilis forms a redox relay with the soluble periplasmic protein PmScsC. We also found that the periplasmic α-domain is sufficient to activate the disulfide isomerase activity of PmScsC. The crystal structure of PmScsBα at a resolution of 1.54 Å revealed that it comprises two structurally similar immunoglobulin-like folds, one of which includes a putative redox-active site with the sequence CXXXC. We confirmed the importance of these cysteine residues for PmScsBα function, and in addition, we engineered cysteine variants that produced a stable complex between PmScsC and PmScsBα. Using small-angle X-ray and neutron scattering analyses with contrast variation, we determined a low-resolution structure of the PmScsC-PmScsBα complex. The structural model of this complex suggested that PmScsBα uses both of its immunoglobulin-like folds to interact with PmScsC and revealed that the highly dynamic PmScsC becomes ordered upon PmScsBα binding. These findings add to our understanding of the poorly characterized Scs proteins.
Keywords: CXXXC active site; Scs protein; bacterial copper sensitivity; copper; disulfide bond; immunoglobulin-like domain; oxidation-reduction (redox); protein disulfide isomerase; protein structure; thioredoxin fold.
© 2018 Furlong et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
The suppressor of copper sensitivity protein C from Caulobacter crescentus is a trimeric disulfide isomerase that binds copper(I) with subpicomolar affinity.Acta Crystallogr D Struct Biol. 2022 Mar 1;78(Pt 3):337-352. doi: 10.1107/S2059798322000729. Epub 2022 Feb 21. Acta Crystallogr D Struct Biol. 2022. PMID: 35234148 Free PMC article.
-
Engineered variants provide new insight into the structural properties important for activity of the highly dynamic, trimeric protein disulfide isomerase ScsC from Proteus mirabilis.Acta Crystallogr D Struct Biol. 2019 Mar 1;75(Pt 3):296-307. doi: 10.1107/S2059798319000081. Epub 2019 Feb 26. Acta Crystallogr D Struct Biol. 2019. PMID: 30950400 Free PMC article.
-
Expression, purification and characterization of the suppressor of copper sensitivity (Scs) B membrane protein from Proteus mirabilis.Protein Expr Purif. 2022 May;193:106047. doi: 10.1016/j.pep.2022.106047. Epub 2022 Jan 11. Protein Expr Purif. 2022. PMID: 35026386
-
Protein disulfides and protein disulfide oxidoreductases in hyperthermophiles.FEBS J. 2006 Sep;273(18):4170-85. doi: 10.1111/j.1742-4658.2006.05421.x. Epub 2006 Aug 23. FEBS J. 2006. PMID: 16930136 Review.
-
Pathways of disulfide bond formation in Escherichia coli.Int J Biochem Cell Biol. 2006;38(7):1050-62. doi: 10.1016/j.biocel.2005.12.011. Epub 2006 Jan 11. Int J Biochem Cell Biol. 2006. PMID: 16446111 Review.
Cited by
-
Protein Disulfide Exchange by the Intramembrane Enzymes DsbB, DsbD, and CcdA.J Mol Biol. 2020 Aug 21;432(18):5091-5103. doi: 10.1016/j.jmb.2020.04.008. Epub 2020 Apr 16. J Mol Biol. 2020. PMID: 32305461 Free PMC article. Review.
-
The suppressor of copper sensitivity protein C from Caulobacter crescentus is a trimeric disulfide isomerase that binds copper(I) with subpicomolar affinity.Acta Crystallogr D Struct Biol. 2022 Mar 1;78(Pt 3):337-352. doi: 10.1107/S2059798322000729. Epub 2022 Feb 21. Acta Crystallogr D Struct Biol. 2022. PMID: 35234148 Free PMC article.
-
CpxR/CpxA Controls scsABCD Transcription To Counteract Copper and Oxidative Stress in Salmonella enterica Serovar Typhimurium.J Bacteriol. 2018 Jul 25;200(16):e00126-18. doi: 10.1128/JB.00126-18. Print 2018 Aug 15. J Bacteriol. 2018. PMID: 29866803 Free PMC article.
-
Stress control for a well-structured life.J Biol Chem. 2018 Apr 20;293(16):5806-5807. doi: 10.1074/jbc.H118.002699. J Biol Chem. 2018. PMID: 29678889 Free PMC article.
-
Engineered variants provide new insight into the structural properties important for activity of the highly dynamic, trimeric protein disulfide isomerase ScsC from Proteus mirabilis.Acta Crystallogr D Struct Biol. 2019 Mar 1;75(Pt 3):296-307. doi: 10.1107/S2059798319000081. Epub 2019 Feb 26. Acta Crystallogr D Struct Biol. 2019. PMID: 30950400 Free PMC article.
References
-
- Cho S. H., Parsonage D., Thurston C., Dutton R. J., Poole L. B., Collet J. F., and Beckwith J. (2012) A new family of membrane electron transporters and its substrates, including a new cell envelope peroxiredoxin, reveal a broadened reductive capacity of the oxidative bacterial cell envelope. mBio 3, e00291–11 10.1128/mBio.00291-11 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

