Unique Phenotypic Characteristics of Recently Transmitted HIV-1 Subtype C Envelope Glycoprotein gp120: Use of CXCR6 Coreceptor by Transmitted Founder Viruses
- PMID: 29491151
- PMCID: PMC5899188
- DOI: 10.1128/JVI.00063-18
Unique Phenotypic Characteristics of Recently Transmitted HIV-1 Subtype C Envelope Glycoprotein gp120: Use of CXCR6 Coreceptor by Transmitted Founder Viruses
Abstract
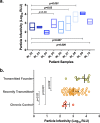
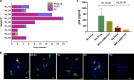
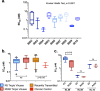
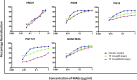
Adequate information on the precise molecular and biological composition of the viral strains that establish HIV infection in the human host will provide effective means of immunization against HIV infection. In an attempt to identify the transmitted founder (TF) virus and differentiate the biological properties and infectious potential of the TF virus from those of the population of the early transmitted viruses, 250 patient-derived gp120 envelope glycoproteins were cloned in pMN-K7-Luc-IRESs-NefΔgp120 to obtain chimeric viruses. Samples were obtained from eight infants who had recently become infected with HIV through mother-to-child transmission (MTCT) and two adults who acquired infection through the heterosexual route and were in the chronic stage of infection. Among the 250 clones tested, 65 chimeric viruses were infectious, and all belonged to HIV-1 subtype C. The 65 clones were analyzed for molecular features of the envelope, per-infectious-particle infectivity, coreceptor tropism, drug sensitivity, and sensitivity to broadly neutralizing antibodies. Based on genotypic and phenotypic analysis of the viral clones, we identified 10 TF viruses from the eight infants. The TF viruses were characterized by shorter V1V2 regions, a reduced number of potential N-linked glycosylation sites, and a higher infectivity titer compared to the virus variants from the adults in the chronic stage of infection. CXCR6 coreceptor usage, in addition to that of the CCR5 coreceptor, which was used by all 65 chimeric viruses, was identified in 13 viruses. The sensitivity of the TF variants to maraviroc and a standard panel of neutralizing monoclonal antibodies (VRC01, PG09, PG16, and PGT121) was found to be much lower than that of the virus variants from the adults in the chronic stage of infection.IMPORTANCE Tremendous progress has been made during the last three and half decades of HIV research, but some significant gaps continue to exist. One of the frontier areas of HIV research which has not seen a breakthrough yet is vaccine research, which is because of the enormous genetic diversity of HIV-1 and the unique infectious fitness of the virus. Among the repertoire of viral variants, the virus that establishes successful infection (transmitted founder [TF] virus) has not been well characterized yet. An insight into the salient features of the TF virus would go a long way toward helping with the design of an effective vaccine against HIV. Here we studied the biological properties of recently transmitted viruses isolated from infants who acquired infection from the mother and have come up with unique characterizations for the TF virus that establishes infection in the human host.
Keywords: amino acid diversity; coreceptor tropism; human immunodeficiency virus; mother-to-child transmission; resistance to neutralization; transmitted founder virus.
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Frequency and Env determinants of HIV-1 subtype C strains from antiretroviral therapy-naive subjects that display incomplete inhibition by maraviroc.Retrovirology. 2016 Nov 3;13(1):74. doi: 10.1186/s12977-016-0309-2. Retrovirology. 2016. PMID: 27809912 Free PMC article.
-
HIV-1 dual/mixed tropic isolates show different genetic and phenotypic characteristics and response to maraviroc in vitro.Antiviral Res. 2011 Apr;90(1):42-53. doi: 10.1016/j.antiviral.2011.02.005. Epub 2011 Feb 22. Antiviral Res. 2011. PMID: 21349294
-
Envelope variants from women recently infected with clade A human immunodeficiency virus type 1 confer distinct phenotypes that are discerned by competition and neutralization experiments.J Virol. 2003 Aug;77(15):8448-61. doi: 10.1128/jvi.77.15.8448-8461.2003. J Virol. 2003. PMID: 12857914 Free PMC article.
-
Effect of HIV-1 subtype and tropism on treatment with chemokine coreceptor entry inhibitors; overview of viral entry inhibition.Crit Rev Microbiol. 2015;41(4):473-87. doi: 10.3109/1040841X.2013.867829. Epub 2014 Mar 17. Crit Rev Microbiol. 2015. PMID: 24635642 Review.
-
HIV-1 subtype C predicted co-receptor tropism in Africa: an individual sequence level meta-analysis.AIDS Res Ther. 2020 Feb 7;17(1):5. doi: 10.1186/s12981-020-0263-x. AIDS Res Ther. 2020. PMID: 32033571 Free PMC article. Review.
Cited by
-
Understanding the mechanisms driving the spread of subtype C HIV-1.EBioMedicine. 2020 Mar;53:102682. doi: 10.1016/j.ebiom.2020.102682. Epub 2020 Feb 27. EBioMedicine. 2020. PMID: 32114391 Free PMC article. Review.
-
Deciphering DNA Methylation in HIV Infection.Front Immunol. 2021 Dec 2;12:795121. doi: 10.3389/fimmu.2021.795121. eCollection 2021. Front Immunol. 2021. PMID: 34925380 Free PMC article. Review.
-
To prescreen or not to prescreen for broadly neutralizing antibody sensitivity in HIV cure-related trials.J Virus Erad. 2023 Jul 18;9(3):100339. doi: 10.1016/j.jve.2023.100339. eCollection 2023 Sep. J Virus Erad. 2023. PMID: 37692548 Free PMC article.
-
Variable infectivity and conserved engagement in cell-to-cell viral transfer by HIV-1 Env from Clade B transmitted founder clones.Virology. 2019 Jan 2;526:189-202. doi: 10.1016/j.virol.2018.10.016. Epub 2018 Nov 8. Virology. 2019. PMID: 30415130 Free PMC article.
-
Potent Induction of Envelope-Specific Antibody Responses by Virus-Like Particle Immunogens Based on HIV-1 Envelopes from Patients with Early Broadly Neutralizing Responses.J Virol. 2022 Jan 12;96(1):e0134321. doi: 10.1128/JVI.01343-21. Epub 2021 Oct 20. J Virol. 2022. PMID: 34668778 Free PMC article.
References
-
- Rong R, Li B, Lynch RM, Haaland RE, Murphy MK, Mulenga J, Allen SA, Pinter A, Shaw GM, Hunter E, Robinson JE, Gnanakaran S, Derdeyn CA. 2009. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog 5:e1000594. doi: 10.1371/journal.ppat.1000594. - DOI - PMC - PubMed
-
- Ferre AL, Hunt PW, Critchfield JW, Young DH, Morris MM, Garcia JC, Pollard RB, Yee HF Jr, Martin JN, Deeks SG, Shacklett BL. 2009. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood 113:3978–3989. doi: 10.1182/blood-2008-10-182709. - DOI - PMC - PubMed
-
- Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi: 10.1038/nature11544. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

