An M2 Rather than a TH2 Response Contributes to Better Protection against Latency Reactivation following Ocular Infection of Naive Mice with a Recombinant Herpes Simplex Virus 1 Expressing Murine Interleukin-4
- PMID: 29491152
- PMCID: PMC5923089
- DOI: 10.1128/JVI.00051-18
An M2 Rather than a TH2 Response Contributes to Better Protection against Latency Reactivation following Ocular Infection of Naive Mice with a Recombinant Herpes Simplex Virus 1 Expressing Murine Interleukin-4
Abstract
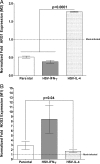
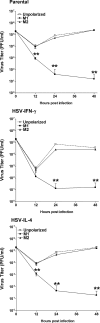
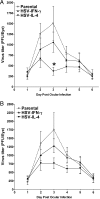
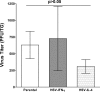
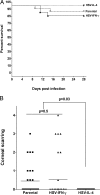
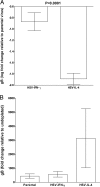
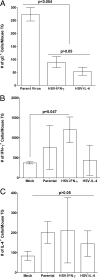
We found previously that altering macrophage polarization toward M2 responses by injection of colony-stimulating factor 1 (CSF-1) was more effective in reducing both primary and latent infections in mice ocularly infected with herpes simplex virus 1 (HSV-1) than M1 polarization by gamma interferon (IFN-γ) injection. Cytokines can coordinately regulate macrophage and T helper (TH) responses, with interleukin-4 (IL-4) inducing type 2 TH (TH2) as well as M2 responses and IFN-γ inducing TH1 as well as M1 responses. We have now differentiated the contributions of these immune compartments to protection against latency reactivation and corneal scarring by comparing the effects of infection with recombinant HSV-1 in which the latency-associated transcript (LAT) gene was replaced with either the IL-4 (HSV-IL-4) or IFN-γ (HSV-IFN-γ) gene using infection with the parental (LAT-negative) virus as a control. Analysis of peritoneal macrophages in vitro established that the replacement of LAT with the IL-4 or IFN-γ gene did not affect virus infectivity and promoted polarization appropriately. Protection against corneal scarring was significantly higher in mice ocularly infected with HSV-IL-4 than in those infected with HSV-IFN-γ or parental virus. Levels of primary virus replication in the eyes and trigeminal ganglia (TG) were similar in the three groups of mice, but the numbers of gC+ cells were lower on day 5 postinfection in the eyes of HSV-IL-4-infected mice than in those infected with HSV-IFN-γ or parental virus. Latency and explant reactivation were lower in both HSV-IL-4- and HSV-IFN-γ-infected mice than in those infected with parental virus, with the lowest level of latency being associated with HSV-IL-4 infection. Higher latency correlated with higher levels of CD8, PD-1, and IFN-γ mRNA, while reduced latency and T-cell exhaustion correlated with lower gC+ expression in the TG. Depletion of macrophages increased the levels of latency in all ocularly infected mice compared with their undepleted counterparts, with macrophage depletion increasing latency in the HSV-IL-4 group greater than 3,000-fold. Our results suggest that shifting the innate macrophage immune responses toward M2, rather than M1, responses in HSV-1 infection would improve protection against establishment of latency, reactivation, and eye disease.IMPORTANCE Ocular HSV-1 infections are among the most frequent serious viral eye infections in the United States and a major cause of virus-induced blindness. As establishment of a latent infection in the trigeminal ganglia results in recurrent infection and is associated with corneal scarring, prevention of latency reactivation is a major therapeutic goal. It is well established that absence of latency-associated transcripts (LATs) reduces latency reactivation. Here we demonstrate that recombinant HSV-1 expressing IL-4 (an inducer of TH2/M2 responses) or IFN-γ (an inducer of TH1/M1 responses) in place of LAT further reduced latency, with HSV-IL-4 showing the highest overall protective efficacy. In naive mice, this higher protective efficacy was mediated by innate rather than adaptive immune responses. Although both M1 and M2 macrophage responses were protective, shifting macrophages toward an M2 response through expression of IL-4 was more effective in curtailing ocular HSV-1 latency reactivation.
Keywords: IFN-γ; IL-4; T cells; eye disease; herpes simplex virus; latency; macrophages; ocular; ocular infection; reactivation; recombinant viruses; trigeminal ganglia.
Copyright © 2018 American Society for Microbiology.
Figures



Similar articles
-
Infection of BALB/c mice with a herpes simplex virus type 1 recombinant virus expressing IFN-gamma driven by the LAT promoter.Virology. 2002 Oct 10;302(1):144-54. doi: 10.1006/viro.2002.1609. Virology. 2002. PMID: 12429523
-
Roles of M1 and M2 Macrophages in Herpes Simplex Virus 1 Infectivity.J Virol. 2017 Jul 12;91(15):e00578-17. doi: 10.1128/JVI.00578-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28490589 Free PMC article.
-
IFNβ absence compensates for LAT functions in latency reactivation and T cell exhaustion.J Virol. 2025 Jun 17;99(6):e0037425. doi: 10.1128/jvi.00374-25. Epub 2025 May 12. J Virol. 2025. PMID: 40353667 Free PMC article.
-
[Battle with herpes for 37 years].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):145-66; discussion 167. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854108 Review. Japanese.
-
Control of HSV-1 latency in human trigeminal ganglia--current overview.J Neurovirol. 2011 Dec;17(6):518-27. doi: 10.1007/s13365-011-0063-0. Epub 2011 Dec 3. J Neurovirol. 2011. PMID: 22139603 Review.
Cited by
-
Understanding the interplay between oHSV and the host immune system: Implications for therapeutic oncolytic virus development.Mol Ther. 2025 Apr 2;33(4):1327-1343. doi: 10.1016/j.ymthe.2024.12.054. Epub 2024 Dec 30. Mol Ther. 2025. PMID: 39741405 Review.
-
Absence of Signal Peptide Peptidase, an Essential Herpes Simplex Virus 1 Glycoprotein K Binding Partner, Reduces Virus Infectivity In Vivo.J Virol. 2019 Nov 13;93(23):e01309-19. doi: 10.1128/JVI.01309-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511378 Free PMC article.
-
Expression of Murine CD80 by Herpes Simplex Virus 1 in Place of Latency-Associated Transcript (LAT) Can Compensate for Latency Reactivation and Anti-apoptotic Functions of LAT.J Virol. 2020 Feb 28;94(6):e01798-19. doi: 10.1128/JVI.01798-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31852788 Free PMC article.
-
A Journey through the Minefield of the Discovery and Characterization of Latency-Related RNA/Latency-Associated Transcript.Viruses. 2024 Sep 30;16(10):1562. doi: 10.3390/v16101562. Viruses. 2024. PMID: 39459896 Free PMC article. Review.
-
Multifunctional Non-Coding RNAs Mediate Latent Infection and Recurrence of Herpes Simplex Viruses.Infect Drug Resist. 2021 Dec 14;14:5335-5349. doi: 10.2147/IDR.S334769. eCollection 2021. Infect Drug Resist. 2021. PMID: 34934329 Free PMC article. Review.
References
-
- Wilhelmus KR, Dawson CR, Barron BA, Bacchetti P, Gee L, Jones DB, Kaufman HE, Sugar J, Hyndiuk RA, Laibson PR, Stulting RD, Asbell PA. 1996. Risk factors for herpes simplex virus epithelial keratitis recurring during treatment of stromal keratitis or iridocyclitis. Herpetic Eye Disease Study Group. Br J Ophthalmol 80:969–972. - PMC - PubMed
-
- Binder PS. 1984. A review of the treatment of ocular herpes simplex infections in the neonate and immunocompromised host. Cornea 3:178–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

