Mechanical circulatory support device-heart hysteretic interaction can predict left ventricular end diastolic pressure
- PMID: 29491185
- PMCID: PMC6530904
- DOI: 10.1126/scitranslmed.aao2980
Mechanical circulatory support device-heart hysteretic interaction can predict left ventricular end diastolic pressure
Abstract
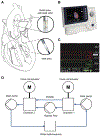
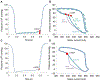
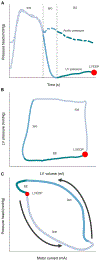
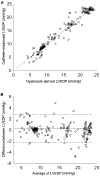
The full potential of mechanical circulatory systems in the treatment of cardiogenic shock is impeded by the lack of accurate measures of cardiac function to guide clinicians in determining when to initiate and how to optimally titrate support. The left ventricular end diastolic pressure (LVEDP) is an established metric of cardiac function that refers to the pressure in the left ventricle at the end of ventricular filling and immediately before ventricular contraction. In clinical practice, LVEDP is typically only inferred from, and poorly correlates with, the pulmonary capillary wedge pressure (PCWP). We leveraged the position of an indwelling percutaneous ventricular assist device and advanced data analysis methods to obtain LVEDP from the hysteretic operating metrics of the device. We validated our hysteresis-derived LVEDP measurement using mock flow loops, an animal model of cardiac dysfunction, and data from a patient in cardiogenic shock to show greater measurement precision and correlation with actual pressures than traditional inferences via PCWP. Delineation of the nonlinear relationship between device and heart adds insight into the interaction between ventricular support devices and the native heart, paving the way for continuous assessment of underlying cardiac state, metrics of cardiac function, potential closed-loop automated control, and rational design of future innovations in mechanical circulatory support systems.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

Similar articles
-
Poor agreement between pulmonary capillary wedge pressure and left ventricular end-diastolic pressure in a veteran population.PLoS One. 2014 Jan 31;9(1):e87304. doi: 10.1371/journal.pone.0087304. eCollection 2014. PLoS One. 2014. PMID: 24498069 Free PMC article.
-
Usefulness of pulmonary capillary wedge pressure as a correlate of left ventricular filling pressures in pulmonary arterial hypertension.J Heart Lung Transplant. 2014 Feb;33(2):157-62. doi: 10.1016/j.healun.2013.10.008. Epub 2013 Oct 11. J Heart Lung Transplant. 2014. PMID: 24268673
-
HVAD Waveform Analysis as a Noninvasive Marker of Pulmonary Capillary Wedge Pressure: A First Step Toward the Development of a Smart Left Ventricular Assist Device Pump.ASAIO J. 2018 Jan/Feb;64(1):10-15. doi: 10.1097/MAT.0000000000000604. ASAIO J. 2018. PMID: 28604571 Free PMC article.
-
"Left ventricular filling pressure(s)" - Ambiguous and misleading terminology, best abandoned.Int J Cardiol. 2015 Jul 15;191:110-3. doi: 10.1016/j.ijcard.2015.04.254. Epub 2015 May 1. Int J Cardiol. 2015. PMID: 25965616 Review.
-
Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock.Circ Cardiovasc Interv. 2017 May;10(5):e004337. doi: 10.1161/CIRCINTERVENTIONS.116.004337. Circ Cardiovasc Interv. 2017. PMID: 28500136 Free PMC article. Review.
Cited by
-
A Scalable Approach to Determine Intracardiac Pressure From Mechanical Circulatory Support Device Signals.IEEE Trans Biomed Eng. 2021 Mar;68(3):905-913. doi: 10.1109/TBME.2020.3016220. Epub 2021 Feb 18. IEEE Trans Biomed Eng. 2021. PMID: 32784129 Free PMC article.
-
Dynamic Modulation of Device-Arterial Coupling to Determine Cardiac Output and Vascular Resistance.Ann Biomed Eng. 2020 Sep;48(9):2333-2342. doi: 10.1007/s10439-020-02510-3. Epub 2020 Apr 13. Ann Biomed Eng. 2020. PMID: 32285344 Free PMC article.
-
Dynamic load modulation predicts right heart tolerance of left ventricular cardiovascular assist in a porcine model of cardiogenic shock.Sci Transl Med. 2024 Feb 14;16(734):eadk4266. doi: 10.1126/scitranslmed.adk4266. Epub 2024 Feb 14. Sci Transl Med. 2024. PMID: 38354226 Free PMC article.
-
Leveraging Device-Arterial Coupling to Determine Cardiac and Vascular State.IEEE Trans Biomed Eng. 2019 Oct;66(10):2800-2808. doi: 10.1109/TBME.2019.2895752. Epub 2019 Jan 28. IEEE Trans Biomed Eng. 2019. PMID: 30703007 Free PMC article.
-
Steady Flow Left Ventricle Unloading Is Superior to Pulsatile Pressure Augmentation Venting During Venoarterial Extracorporeal Membrane Oxygenation Support.ASAIO J. 2024 Nov 1;70(11):929-937. doi: 10.1097/MAT.0000000000002208. Epub 2024 Apr 8. ASAIO J. 2024. PMID: 38588597
References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation 133, e38–e360 (2016). - PubMed
-
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ, Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 123, 933–944 (2011). - PubMed
-
- Reyentovich A, Barghash MH, Hochman JS, Management of refractory cardiogenic shock. Nat. Rev. Cardiol. 13, 481–492 (2016). - PubMed
-
- De Luca L, Olivari Z, Farina A, Gonzini L, Lucci D, Di Chiara A, Casella G, Chiarella F, Boccanelli A, Di Pasquale G, De Servi S, Bovenzi FM, Gulizia MM, Savonitto S, Temporal trends in the epidemiology, management, and outcome of patients with cardiogenic shock complicating acute coronary syndromes. Eur. J. Heart Fail. 17, 1124–1132 (2015). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

