Egr2-dependent microRNA-138 is dispensable for peripheral nerve myelination
- PMID: 29491350
- PMCID: PMC5830491
- DOI: 10.1038/s41598-018-22010-8
Egr2-dependent microRNA-138 is dispensable for peripheral nerve myelination
Abstract
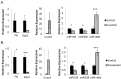
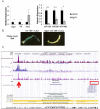
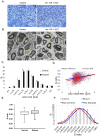
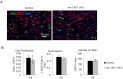
Recent studies have elucidated the crucial role for microRNAs in peripheral nerve myelination by ablating components of the microRNA synthesis machinery. Few studies have focused on the role of individual microRNAs. To fill this gap, we focused this study on miR-138, which was shown to be drastically reduced in Dicer1 and Dgcr8 knockout mice with hypomyelinating phenotypes and to potentially target the negative regulators of Schwann cell differentiation. Here, we show that of two miR-138 encoding loci, mir-138-1 is the predominant locus transcribed in Schwann cells. mir-138-1 is transcriptionally upregulated during myelination and downregulated upon nerve injury. EGR2 is required for mir-138-1 transcription during development, and both SOX10 and EGR2 bind to an active enhancer near the mir-138-1 locus. Based on expression analyses, we hypothesized that miR-138 facilitates the transition between undifferentiated Schwann cells and myelinating Schwann cells. However, in conditional knockouts, we could not detect significant changes in Schwann cell proliferation, cell cycle exit, or myelination. Overall, our results demonstrate that miR-138 is an Egr2-dependent microRNA but is dispensable for Schwann cell myelination.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Ablation of Dicer from murine Schwann cells increases their proliferation while blocking myelination.PLoS One. 2010 Aug 27;5(8):e12450. doi: 10.1371/journal.pone.0012450. PLoS One. 2010. PMID: 20805985 Free PMC article.
-
Genome-wide analysis of EGR2/SOX10 binding in myelinating peripheral nerve.Nucleic Acids Res. 2012 Aug;40(14):6449-60. doi: 10.1093/nar/gks313. Epub 2012 Apr 9. Nucleic Acids Res. 2012. PMID: 22492709 Free PMC article.
-
Microprocessor complex subunit DiGeorge syndrome critical region gene 8 (Dgcr8) is required for schwann cell myelination and myelin maintenance.J Biol Chem. 2015 Oct 2;290(40):24294-307. doi: 10.1074/jbc.M115.636407. Epub 2015 Aug 13. J Biol Chem. 2015. PMID: 26272614 Free PMC article.
-
Regulation of myelin-specific gene expression. Relevance to CMT1.Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review.
-
Regulatory Mechanism of Peripheral Nerve Myelination by Glutamate-Induced Signaling.Adv Exp Med Biol. 2019;1190:23-31. doi: 10.1007/978-981-32-9636-7_2. Adv Exp Med Biol. 2019. PMID: 31760635 Review.
Cited by
-
Crosstalk between MicroRNA and Oxidative Stress in Primary Open-Angle Glaucoma.Int J Mol Sci. 2021 Feb 28;22(5):2421. doi: 10.3390/ijms22052421. Int J Mol Sci. 2021. PMID: 33670885 Free PMC article. Review.
-
How miRNAs Regulate Schwann Cells during Peripheral Nerve Regeneration-A Systemic Review.Int J Mol Sci. 2022 Mar 22;23(7):3440. doi: 10.3390/ijms23073440. Int J Mol Sci. 2022. PMID: 35408800 Free PMC article.
-
Integrative Analysis Identifies Candidate Tumor Microenvironment and Intracellular Signaling Pathways that Define Tumor Heterogeneity in NF1.Genes (Basel). 2020 Feb 21;11(2):226. doi: 10.3390/genes11020226. Genes (Basel). 2020. PMID: 32098059 Free PMC article.
-
MicroRNA-138 Overexpression Alters Aβ42 Levels and Behavior in Wildtype Mice.Front Neurosci. 2021 Jan 14;14:591138. doi: 10.3389/fnins.2020.591138. eCollection 2020. Front Neurosci. 2021. PMID: 33519353 Free PMC article.
-
miRNA Alterations Elicit Pathways Involved in Memory Decline and Synaptic Function in the Hippocampus of Aged Tg4-42 Mice.Front Neurosci. 2020 Sep 10;14:580524. doi: 10.3389/fnins.2020.580524. eCollection 2020. Front Neurosci. 2020. PMID: 33013313 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

