Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages
- PMID: 29491367
- PMCID: PMC5832433
- DOI: 10.1038/s41419-017-0033-4
Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages
Abstract
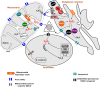
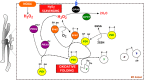
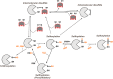
Many cellular redox reactions housed within mitochondria, peroxisomes and the endoplasmic reticulum (ER) generate hydrogen peroxide (H2O2) and other reactive oxygen species (ROS). The contribution of each organelle to the total cellular ROS production is considerable, but varies between cell types and also over time. Redox-regulatory enzymes are thought to assemble at a "redox triangle" formed by mitochondria, peroxisomes and the ER, assembling "redoxosomes" that sense ROS accumulations and redox imbalances. The redoxosome enzymes use ROS, potentially toxic by-products made by some redoxosome members themselves, to transmit inter-compartmental signals via chemical modifications of downstream proteins and lipids. Interestingly, important components of the redoxosome are ER chaperones and oxidoreductases, identifying ER oxidative protein folding as a key ROS producer and controller of the tri-organellar membrane contact sites (MCS) formed at the redox triangle. At these MCS, ROS accumulations could directly facilitate inter-organellar signal transmission, using ROS transporters. In addition, ROS influence the flux of Ca2+ ions, since many Ca2+ handling proteins, including inositol 1,4,5 trisphosphate receptors (IP3Rs), SERCA pumps or regulators of the mitochondrial Ca2+ uniporter (MCU) are redox-sensitive. Fine-tuning of these redox and ion signaling pathways might be difficult in older organisms, suggesting a dysfunctional redox triangle may accompany the aging process.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

