PAN-cancer analysis of S-phase enriched lncRNAs identifies oncogenic drivers and biomarkers
- PMID: 29491376
- PMCID: PMC5830406
- DOI: 10.1038/s41467-018-03265-1
PAN-cancer analysis of S-phase enriched lncRNAs identifies oncogenic drivers and biomarkers
Abstract
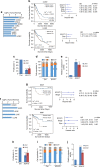
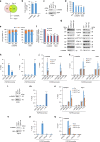
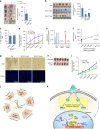
Despite improvement in our understanding of long noncoding RNAs (lncRNAs) role in cancer, efforts to find clinically relevant cancer-associated lncRNAs are still lacking. Here, using nascent RNA capture sequencing, we identify 1145 temporally expressed S-phase-enriched lncRNAs. Among these, 570 lncRNAs show significant differential expression in at least one tumor type across TCGA data sets. Systematic clinical investigation of 14 Pan-Cancer data sets identified 633 independent prognostic markers. Silencing of the top differentially expressed and clinically relevant S-phase-enriched lncRNAs in several cancer models affects crucial cancer cell hallmarks. Mechanistic investigations on SCAT7 in multiple cancer types reveal that it interacts with hnRNPK/YBX1 complex and affects cancer cell hallmarks through the regulation of FGF/FGFR and its downstream PI3K/AKT and MAPK pathways. We also implement a LNA-antisense oligo-based strategy to treat cancer cell line and patient-derived tumor (PDX) xenografts. Thus, this study provides a comprehensive list of lncRNA-based oncogenic drivers with potential prognostic value.
Conflict of interest statement
C.K., M.M.A., V.S.A., S.T.K., S.S. and L.S. have filed a provisional patent (no:1750724-5/160080SE) titled “Long noncoding RNA in Cancer” based on findings from the current manuscript. The remaining authors declare no competing interests.
Figures





Comment in
-
S-phase cancer associated lncRNAs.Cell Cycle. 2018;17(23):2517-2519. doi: 10.1080/15384101.2018.1553356. Epub 2018 Dec 3. Cell Cycle. 2018. PMID: 30482083 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

