Rapid Detection of Zika Virus in Urine Samples and Infected Mosquitos by Reverse Transcription-Loop-Mediated Isothermal Amplification
- PMID: 29491389
- PMCID: PMC5830622
- DOI: 10.1038/s41598-018-22102-5
Rapid Detection of Zika Virus in Urine Samples and Infected Mosquitos by Reverse Transcription-Loop-Mediated Isothermal Amplification
Abstract
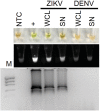
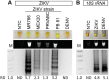
Infection with Zika virus (ZIKV) is of growing concern since infection is associated with the development of congenital neurological disease. Quantitative reverse transcription PCR (qRT-PCR) has been the standard for ZIKV detection; however, Reverse Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) may allow for faster and cheaper testing. Studies have suggested that ZIKV detection in urine is more sensitive and has a longer window of detection compared to serum and saliva. The objective of this study was to develop a urine diagnostic test that could be completed in under 30 minutes. Urine samples spiked with ZIKV or dengue virus were tested using RT-LAMP as well as by conventional quantitative qRT-PCR. These techniques were then validated using crude lysates made from ZIKV infected mosquitoes in addition to urine and serum samples from ZIKV infected patients. RT-LAMP specifically detected ZIKV in urine and serum for ZIKV infected patients and crude mosquito lysates. This test was performed in under 30 minutes and did not require RNA extraction from urine nor mosquitos. This approach could be used for monitoring of exposed individuals, especially pregnant women, couples wanting to conceive, or individuals with suspicious symptoms as well as surveillance of mosquito populations.
Conflict of interest statement
L.E.L. and M.C. have intellectual property on Zika virus diagnosis methods. The remaining authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

