FcαRI co-stimulation converts human intestinal CD103+ dendritic cells into pro-inflammatory cells through glycolytic reprogramming
- PMID: 29491406
- PMCID: PMC5830413
- DOI: 10.1038/s41467-018-03318-5
FcαRI co-stimulation converts human intestinal CD103+ dendritic cells into pro-inflammatory cells through glycolytic reprogramming
Abstract
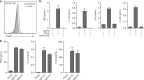
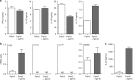
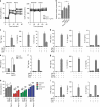
CD103+ dendritic cells (DC) are crucial for regulation of intestinal tolerance in humans. However, upon infection of the lamina propria this tolerogenic response is converted to an inflammatory response. Here we show that immunoglobulin A (IgA) immune complexes (IgA-IC), which are present after bacterial infection of the lamina propria, are important for the induction of inflammation by the human CD103+SIRPα+ DC subset. IgA-IC, by recognition through FcαRI, selectively amplify the production of proinflammatory cytokines TNF, IL-1β and IL-23 by human CD103+ DCs. These cells then enhance inflammation by promoting Th17 responses and activating human intestinal innate lymphoid cells 3. Moreover, FcαRI-induced cytokine production is orchestrated via upregulation of cytokine translation and caspase-1 activation, which is dependent on glycolytic reprogramming mediated by kinases Syk, PI3K and TBK1-IKKε. Our data suggest that the formation of IgA-IC in the human intestine provides an environmental cue for the conversion of a tolerogenic to an inflammatory response.
Conflict of interest statement
D.L.P.B. is a part-time employee of UCB. G.R.v.d.B. is currently employed by GlaxoSmithKline. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

