New thin-film surface electrode array enables brain mapping with high spatial acuity in rodents
- PMID: 29491453
- PMCID: PMC5830616
- DOI: 10.1038/s41598-018-22051-z
New thin-film surface electrode array enables brain mapping with high spatial acuity in rodents
Abstract
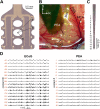
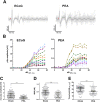
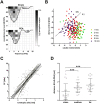
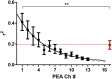
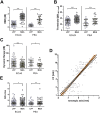
In neuroscience, single-shank penetrating multi-electrode arrays are standard for sequentially sampling several cortical sites with high spatial and temporal resolution, with the disadvantage of neuronal damage. Non-penetrating surface grids used in electrocorticography (ECoG) permit simultaneous recording of multiple cortical sites, with limited spatial resolution, due to distance to neuronal tissue, large contact size and high impedances. Here we compared new thin-film parylene C ECoG grids, covering the guinea pig primary auditory cortex, with simultaneous recordings from penetrating electrode array (PEAs), inserted through openings in the grid material. ECoG grid local field potentials (LFP) showed higher response thresholds and amplitudes compared to PEAs. They enabled, however, fast and reliable tonotopic mapping of the auditory cortex (place-frequency slope: 0.7 mm/octave), with tuning widths similar to PEAs. The ECoG signal correlated best with supragranular layers, exponentially decreasing with cortical depth. The grids also enabled recording of multi-unit activity (MUA), yielding several advantages over LFP recordings, including sharper frequency tunings. ECoG first spike latency showed highest similarity to superficial PEA contacts and MUA traces maximally correlated with PEA recordings from the granular layer. These results confirm high quality of the ECoG grid recordings and the possibility to collect LFP and MUA simultaneously.
Conflict of interest statement
At the time of the ECoG development, U.P.F. was employed by Blackrock Microsystems Europe. The authors declare no further conflict of interest. This project was supported by the German Federal Ministry of Education and Research KMU-Innovativ: Medizintechnik FKZ 13GW0050B and by the Deutsche Forschungsgemeinschaft (DFG) EXC 1077/1 Hearing4all. Other than providing financial support, the funding sources had no part in the study.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

