Functional intercalated nanocomposites with chitosan-glutathione-glycylsarcosine and layered double hydroxides for topical ocular drug delivery
- PMID: 29491707
- PMCID: PMC5815481
- DOI: 10.2147/IJN.S148104
Functional intercalated nanocomposites with chitosan-glutathione-glycylsarcosine and layered double hydroxides for topical ocular drug delivery
Abstract
Background: To enhance ocular bioavailability, the traditional strategies have focused on prolonging precorneal retention and improving corneal permeability by nano-carriers with positive charge, thiolated polymer, absorption enhancer and so on. Glycylsarcosine (GS) as an active target ligand of the peptide tranpsporter-1 (PepT-1), could specific interact with the PepT-1 on the cornea and guide the nanoparticles to the treating site.
Purpose: The objective of the study was to explore the active targeting intercalated nanocomposites based on chitosan-glutathione-glycylsarcosine (CG-GS) and layered double hydroxides (LDH) as novel carriers for the treatment of mid-posterior diseases.
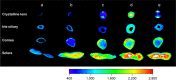
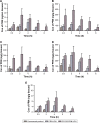
Materials and methods: CG-GS-LDH intercalated nanocomposites were prepared by the coprecipitation hydrothermal method. In vivo precorneal retention study, ex vivo fluorescence images, in vivo experiment for distribution and irritation were studied in rabbits. The cytotoxicity and cellular uptake were studied in human corneal epithelial primary cells (HCEpiC).
Results: CG-GS-LDH nanocomposites were prepared successfully and characterized by FTIR and XRD. Experiments with rabbits showed longer precorneal retention and higher distribution of fluorescence probe/model drug. In vitro cytological study, CG-GS-LDH nanocomposites exhibited enhanced cellular uptake compared to pure drug solution. Furthermore, the investigation of cellular uptake mechanisms demonstrated that both the active transport by PepT-1 and clathrin-mediated endocytosis were involved in the internalization of CG-GS-LDH intercalated nanocomposites. An ocular irritation study and a cytotoxicity test indicated that these nanocomposites produced no significant irritant effects.
Conclusions: The active targeting intercalated nanocomposites could have great potential for topical ocular drug delivery due to the capacity for prolonging the retention on the ocular surface, enhancing the drug permeability through the cornea, and efficiently delivering the drug to the targeted site.
Keywords: active targeting; glycylsarcosine; intercalated nanocomposites; layered double hydroxides; peptide transporter-1.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures








References
-
- Kawashima T, Nagai N, Kaji H, et al. A scalable controlled-release device for transscleral drug delivery to the retina. Biomaterials. 2011;32(7):1950–1956. - PubMed
-
- Lai JY, Hsieh AC. A gelatin-g-poly(N-isopropylacrylamide) biodegradable in situ gelling delivery system for the intracameral administration of pilocarpine. Biomaterials. 2012;33(7):2372–2387. - PubMed
-
- Lai PX, Chen CW, Wei SC, et al. Ultrastrong trapping of VEGF by graphene oxide: anti-angiogenesis application. Biomaterials. 2016;109(Suppl C):12–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

