Zinc ion dyshomeostasis increases resistance of prostate cancer cells to oxidative stress via upregulation of HIF1α
- PMID: 29492208
- PMCID: PMC5823553
- DOI: 10.18632/oncotarget.23893
Zinc ion dyshomeostasis increases resistance of prostate cancer cells to oxidative stress via upregulation of HIF1α
Abstract
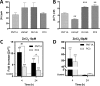
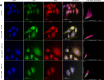
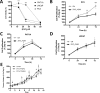
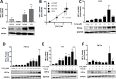
Zinc ions (Zn2+) are known to influence cell survival and proliferation. However the homeostatic regulation of Zn2+ and their role in prostate cancer (PC) progression is poorly understood. Therefore the subcellular distribution and uptake of Zn2+ in PC cells were investigated. Inductively coupled plasma mass spectroscopy and fluorescent microscopy with the Zn2+-specific fluorescent probe FluoZin-3 were used to quantify total and free Zn2+, respectively, in the normal prostate epithelial cell line (PNT1A) and three human PC cell lines (PC3, DU145 and LNCaP). The effects of Zn2+ treatment on proliferation and survival were measured in vitro using MTT assays and in vivo using mouse xenografts. The ability of Zn2+ to protect against oxidative stress via a HIF1α-dependent mechanism was investigated using a HIF1α knock-down PC3 model. Our results demonstrate that the total Zn2+ concentration in normal PNT1A and PC cells is similar, but PC3 cells contain significantly higher free Zn2+ than PNT1A cells (p < 0.01). PNT1A cells can survive better in the presence of high concentrations of Zn2+ than PC3 cells. Exposure to 10 µM Zn2+ over 72 hours significantly reduces PC3 cell proliferation in vitro but not in vivo. Zn2+ increases PC3 cell survival up to 2.3-fold under oxidative stress, and this protective effect is not seen in PNT1A cells or in a HIF1α-KD PC3 cell model. A state of Zn2+ dyshomeostasis exists in PC. HIF1α is an integral component of a Zn2+-dependent protective mechanism present in PC3 cells. This pathway may be clinically significant through its contribution to castrate-resistant PC survival.
Keywords: castrate resistant; hypoxia inducible factor 1 alpha; iron; prostate cancer; zinc.
Conflict of interest statement
CONFLICTS OF INTEREST None.
Figures





Similar articles
-
Monitoring of the prostate tumour cells redox state and real-time proliferation by novel biophysical techniques and fluorescent staining.Integr Biol (Camb). 2012 Jun;4(6):672-84. doi: 10.1039/c2ib00157h. Epub 2012 May 16. Integr Biol (Camb). 2012. PMID: 22592803
-
The role of hypoxia-inducible factor 1α in determining the properties of castrate-resistant prostate cancers.PLoS One. 2013;8(1):e54251. doi: 10.1371/journal.pone.0054251. Epub 2013 Jan 16. PLoS One. 2013. PMID: 23342109 Free PMC article.
-
Stimulating effect of palmitate and insulin on cell migration and proliferation in PNT1A and PC3 prostate cells: Counteracting role of metformin.Prostate. 2018 Jul;78(10):731-742. doi: 10.1002/pros.23517. Epub 2018 Apr 10. Prostate. 2018. PMID: 29635803
-
HIF1α expression under normoxia in prostate cancer--which pathways to target?J Urol. 2015 Mar;193(3):763-70. doi: 10.1016/j.juro.2014.10.085. Epub 2014 Oct 19. J Urol. 2015. PMID: 25444956 Review.
-
Non-genomic effects of the androgen receptor and vitamin D agonist are involved in suppressing invasive phenotype of prostate cancer cells.Steroids. 2006 Apr;71(4):304-9. doi: 10.1016/j.steroids.2005.09.010. Epub 2005 Nov 9. Steroids. 2006. PMID: 16289173 Review.
Cited by
-
The Protective Effect of Zinc Against Liver Ischaemia Reperfusion Injury in a Rat Model of Global Ischaemia.J Clin Exp Hepatol. 2020 May-Jun;10(3):228-235. doi: 10.1016/j.jceh.2019.07.006. Epub 2019 Jul 24. J Clin Exp Hepatol. 2020. PMID: 32405179 Free PMC article.
-
Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics.Int J Mol Sci. 2020 Apr 23;21(8):2991. doi: 10.3390/ijms21082991. Int J Mol Sci. 2020. PMID: 32340289 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

