The role of neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer: a systematic review and meta-analysis of randomized controlled trials and observational studies
- PMID: 29492221
- PMCID: PMC5823572
- DOI: 10.18632/oncotarget.23808
The role of neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer: a systematic review and meta-analysis of randomized controlled trials and observational studies
Abstract
Objective: We aimed to performed a meta-analysis and systematic review on the role of neoadjuvant chemotherapy followed by interval debulking surgery (NACT-IDS) in advanced ovarian cancer (AOC) patients.
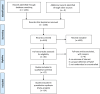
Materials and methods: We searched PubMed, EMBASE, and the Cochrane Library for relevant articles. All statistical analyses were performed in Review Manager 5.3.5.
Results: In two randomized controlled trials (RCTs), there was no significant difference in overall survival (OS) (HR = 0.93, 95% CI: 0.81-1.06) or progression-free survival (PFS) (HR = 0.97, 95% CI: 0.86-1.09). Few adverse events (HR = 0.37, 95% CI: 0.19-0.72) and a high optimal debulking surgery rate (HR = 1.69, 95% CI: 1.50-1.91) were observed with NACT. In 22 observational studies, primary debulking surgery (PDS) yielded better OS (HR = 1.38, 95% CI: 1.19-1.60) but not progression-free survival (PFS) (HR = 1.03, 95% CI: 0.86-1.23). An increased optimal cytoreduction rate (HR = 1.17, 95% CI: 1.12-1.22) was observed with NACT. Irrespective of the degree of residual disease, OS was longer in the PDS group than that in the NACT group. Patients with FIGO stage III (HR = 1.43, 95% CI: 1.05-1.95) and IV (HR = 1.14, 95% CI: 1.06-1.23) disease had better survival with PDS.
Conclusions: Treatment with NACT-IDS improves perioperative outcomes and optimal cytoreduction rates, but it may not improve OS. NACT-IDS is not inferior to PDS-CT in terms of survival outcomes in selected AOC patients. Future studies should focus on candidate selection for NACT.
Keywords: debulking surgery; neoadjuvant chemotherapy; ovarian cancer; survival.
Conflict of interest statement
CONFLICTS OF INTEREST All authors declare that they have no competing interests.
Figures









References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- van Altena AM, Karim-Kos HE, de Vries E, Kruitwagen RF, Massuger LF, Kiemeney LA. Trends in therapy and survival of advanced stage epithelial ovarian cancer patients in the Netherlands. Gynecol Oncol. 2012;125:649–54. - PubMed
-
- Fago-Olsen CL, Ottesen B, Kehlet H, Markauskas A, Mosgaard BJ, Ottosen C, Sogaard CH, Sogaard-Andersen E, Hogdall C. Neoadjuvant chemotherapy as ovarian cancer treatment: ever more used with major regional differences. Dan Med J. 2012;59:A4477. - PubMed
-
- Schorge JO, Clark RM, Lee SI, Penson RT. Primary debulking surgery for advanced ovarian cancer: Are you a believer or a dissenter? Gynecologic Oncology. 2014;135:595–605. - PubMed
-
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

