Current state of pediatric cardiac transplantation
- PMID: 29492382
- PMCID: PMC5827130
- DOI: 10.21037/acs.2018.01.07
Current state of pediatric cardiac transplantation
Abstract
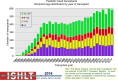
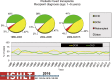
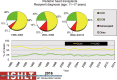
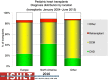
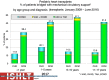
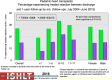
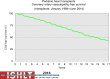
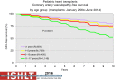
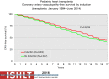
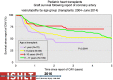
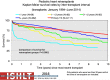
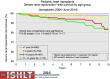
Pediatric heart transplantation is standard of care for children with end-stage heart failure. The diverse age range, diagnoses, and practice variations continue to challenge the development of evidence-based practices and new technologies. Outcomes in the most recent era are excellent, especially with the more widespread use of ventricular assist devices (VADs). Waitlist mortality remains high and knowledge of risk factors for death while waiting and following transplantation contributes to decision-making around transplant candidacy and timing of listing. The biggest gap impacting both waitlist and overall survival remains mechanical support options for infants and patients with single ventricle physiology. Though acute rejection has decreased progressively, both diagnosis and management of antibody-mediated rejection has become increasingly challenging and complex, as has the ability to understand the implication of anti-HLA antibodies detected both pre- and post-transplantation-including when and how to intervene. Trends in immunosuppression protocols include more use of induction therapy and steroid avoidance or withdrawal protocols. Common long-term morbidities include renal insufficiency, which can be mitigated with surveillance and renal-sparing strategies, and infections. Functional outcomes are excellent, but significant psychosocial challenges exist in relation to neurodevelopment, non-adherence, and transition from child-centered to adult-centered care. Cardiac allograft vasculopathy (CAV) remains a barrier to long-term survival, though it is more apparent that objective evidence of an impact on the allograft is important with regards to impact on outcomes. Retransplantation is rare in pediatric heart transplant recipients. Pediatric heart transplantation continues to evolve in order to address the challenges of the diverse group of patients that reach end-stage heart failure during childhood.
Keywords: Heart transplant; mechanical support; outcomes; sensitization; waitlist.
Conflict of interest statement
Conflicts of Interest: The author has no conflicts of interest to declare.
Figures
















References
-
- Rossano JW, Dipchand AI, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Nineteenth Pediatric Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1185-95. 10.1016/j.healun.2016.08.018 - DOI - PubMed
-
- Chambers DC, Yusen RD, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1047-59. 10.1016/j.healun.2017.07.016 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
