Electron Transfer Dissociation and Collision-Induced Dissociation of Underivatized Metallated Oligosaccharides
- PMID: 29492773
- PMCID: PMC5943087
- DOI: 10.1007/s13361-018-1906-1
Electron Transfer Dissociation and Collision-Induced Dissociation of Underivatized Metallated Oligosaccharides
Abstract
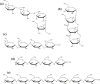
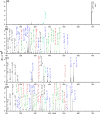
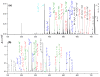
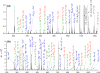
Electron transfer dissociation (ETD) and collision-induced dissociation (CID) were used to investigate underivatized, metal-cationized oligosaccharides formed via electrospray ionization (ESI). Reducing and non-reducing sugars were studied including the tetrasaccharides maltotetraose, 3α,4β,3α-galactotetraose, stachyose, nystose, and a heptasaccharide, maltoheptaose. Univalent alkali, divalent alkaline earth, divalent and trivalent transition metal ions, and a boron group trivalent metal ion were adducted to the non-permethylated oligosaccharides. ESI generated [M + Met]+, [M + 2Met]2+, [M + Met]2+, [M + Met - H]+, and [M + Met - 2H]+ most intensely along with low intensity nitrate adducts, depending on the metal and sugar ionized. The ability of these metal ions to produce oligosaccharide adduct ions by ESI had the general trend: Ca(II) > Mg(II) > Ni(II) > Co(II) > Zn(II) > Cu(II) > Na(I) > K(I) > Al(III) ≈ Fe(III) ≈ Cr(III). Although trivalent metals were utilized, no triply charged ions were formed. Metal cations allowed for high ESI signal intensity without permethylation. ETD and CID on [M + Met]2+ produced various glycosidic and cross-ring cleavages, with ETD producing more cross-ring and internal ions, which are useful for structural analysis. Product ion intensities varied based on glycosidic-bond linkage and identity of monosaccharide sub-unit, and metal adducts. ETD and CID showed high fragmentation efficiency, often with complete precursor dissociation, depending on the identity of the adducted metal ion. Loss of water was occasionally observed, but elimination of small neutral molecules was not prevalent. For both ETD and CID, [M + Co]2+ produced the most uniform structurally informative dissociation with all oligosaccharides studied. The ETD and CID spectra were complementary. Graphical Abstract ᅟ.
Keywords: Carbohydrates; Collision-induced dissociation; Electron transfer dissociation; Metallated oligosacchardies; Oligosaccharides.
Figures




Similar articles
-
In-Source Decay MALDI and High-Energy Collision-Induced Dissociation Mass Spectrometry of Alkali Metal-Adducted Underivatized Oligosaccharides.J Am Soc Mass Spectrom. 2023 Nov 1;34(11):2594-2606. doi: 10.1021/jasms.3c00330. Epub 2023 Oct 9. J Am Soc Mass Spectrom. 2023. PMID: 37812625
-
Effects of transition metal ion coordination on the collision-induced dissociation of polyalanines.J Mass Spectrom. 2011 Nov;46(11):1099-107. doi: 10.1002/jms.1992. J Mass Spectrom. 2011. PMID: 22124980
-
Electron transfer dissociation of milk oligosaccharides.J Am Soc Mass Spectrom. 2011 Jun;22(6):997-1013. doi: 10.1007/s13361-011-0117-9. Epub 2011 Apr 14. J Am Soc Mass Spectrom. 2011. PMID: 21953041 Free PMC article.
-
A review of electron-capture and electron-transfer dissociation tandem mass spectrometry in polymer chemistry.Anal Chim Acta. 2014 Jan 15;808:44-55. doi: 10.1016/j.aca.2013.09.033. Epub 2013 Sep 26. Anal Chim Acta. 2014. PMID: 24370092 Review.
-
Periodic trends in the hydration energies and critical sizes of alkaline earth and transition metal dication water complexes.Mass Spectrom Rev. 2025 Mar-Apr;44(2):135-153. doi: 10.1002/mas.21830. Epub 2023 Jan 16. Mass Spectrom Rev. 2025. PMID: 36644985 Review.
Cited by
-
Structural characterization of human milk oligosaccharides using ultrahigh performance liquid chromatography-helium charge transfer dissociation mass spectrometry.Glycobiology. 2022 May 23;32(6):483-495. doi: 10.1093/glycob/cwac010. Glycobiology. 2022. PMID: 35275172 Free PMC article.
-
Sensitive Fluorescence Quantitation and Efficient Free Radical Characterization of N-Glycans via LC-FLR-HRMS/MS with a Novel Fluorescent Free Radical Tag.Anal Chem. 2025 Apr 8;97(13):7118-7127. doi: 10.1021/acs.analchem.4c06294. Epub 2025 Mar 25. Anal Chem. 2025. PMID: 40129309 Free PMC article.
-
Mechanistic Study into Free Radical-Activated Glycan Dissociations through Isotope-Labeled Cellobioses.Anal Chem. 2023 Feb 7;95(5):2932-2941. doi: 10.1021/acs.analchem.2c04649. Epub 2023 Jan 30. Anal Chem. 2023. PMID: 36715667 Free PMC article.
-
Free-Radical-Mediated Glycan Isomer Differentiation.Anal Chem. 2020 Oct 20;92(20):13794-13802. doi: 10.1021/acs.analchem.0c02213. Epub 2020 Sep 28. Anal Chem. 2020. PMID: 32935980 Free PMC article.
-
Characterization of Glycosphingolipids and Their Diverse Lipid Forms through Two-Stage Matching of LC-MS/MS Spectra.Anal Chem. 2021 Feb 16;93(6):3154-3162. doi: 10.1021/acs.analchem.0c04542. Epub 2021 Feb 3. Anal Chem. 2021. PMID: 33534538 Free PMC article.
References
-
- Parodi AJ. Protein glucosylation and its role in protein folding. Annu. Rev. Biochem. 2000;69:69–93. - PubMed
-
- Fenn JB, Mann M, Meng CK, Wong SF. Electrospray ionization--principles and practice. Mass Spectrom. Rev. 1990;9:37–70.
-
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

