Biokinetic and dosimetric aspects of 64CuCl2 in human prostate cancer: possible theranostic implications
- PMID: 29492782
- PMCID: PMC5833894
- DOI: 10.1186/s13550-018-0373-9
Biokinetic and dosimetric aspects of 64CuCl2 in human prostate cancer: possible theranostic implications
Abstract
Background: The aim of the present study is to evaluate the kinetics and dosimetry of 64CuCl2 in human prostate cancer (PCa) lesions. We prospectively evaluated 50 PCa patients with biochemical relapse after surgery or external beam radiation therapy. All patients underwent 64CuCl2-PET/CT to detect PCa recurrence/metastases. Volumes of interest were manually drawn for each 64CuCl2 avid PCa lesion with a diameter > 1 cm on mpMRI in each patient. Time-activity curves for all lesions were obtained. The effective and biological half-life and the standard uptake values (SUVs) were calculated. Tumour/background ratio (TBR) curves as a function of time were considered. Finally, the absorbed dose per lesion was estimated.
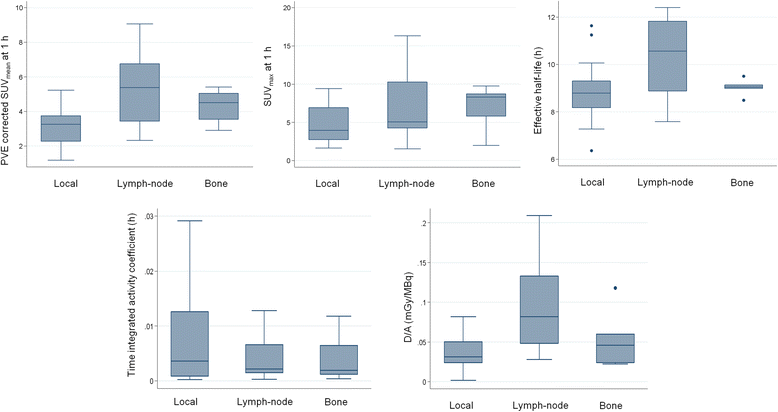
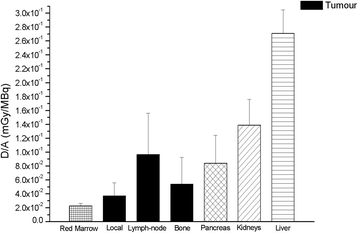
Results: The mean effective half-life of 64CuCl2 calculated in the lymph nodes (10.2 ± 1.7 h) was significantly higher than in local relapses (8.8 ± 1.1 h) and similar to that seen in bone metastases (9.0 ± 0.4 h). The mean 64CuCl2 SUVmax calculated 1 h after tracer injection was significantly higher in the lymph nodes (6.8 ± 4.3) and bone metastases (6.8 ± 2.9) than in local relapses (4.7 ± 2.4). TBR mean curve of 64CuCl2 revealed that the calculated TBRmax value was 5.0, 7.0, and 6.2 in local relapse and lymph node and bone metastases, respectively, and it was achieved about 1 h after 64CuCl2 injection. The mean absorbed dose of the PCa lesions per administrated activity was 6.00E-2 ± 4.74E-2mGy/MBq. Indeed, for an administered activity of 3.7 GBq, the mean dose absorbed by the lesion would be 0.22 Gy.
Conclusions: Dosimetry showed that the dose absorbed by PCa recurrences/metastases per administrated activity was low. The dosimetric study performed does not take into account the possible therapeutic effect of the Auger electrons. Clinical trials are needed to evaluate 64Cu internalization in the cell nucleus that seems related to the therapeutic effectiveness reported in preclinical studies.
Keywords: 64CuCl2; Elderly; Kinetics and dosimetry; Prostate cancer; Theranostic.
Conflict of interest statement
Ethics approval and consent to participate
This protocol was approved by the Bioethical Committee of “Regione Liguria”. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Piccardo A, Paparo F, Puntoni M, et al. 64CuCl2 PET/CT in prostate cancer relapse. J Nucl Med. 2017;8. 10.2967/jnumed.117.195628.
LinkOut - more resources
Full Text Sources
Other Literature Sources

