Frontline Science: Rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages
- PMID: 29493813
- PMCID: PMC5935116
- DOI: 10.1002/JLB.3HI1017-422R
Frontline Science: Rapid adipose tissue expansion triggers unique proliferation and lipid accumulation profiles in adipose tissue macrophages
Abstract
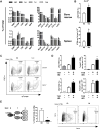
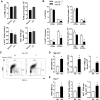
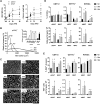
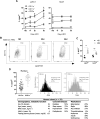
Obesity-related changes in adipose tissue leukocytes, in particular adipose tissue macrophages (ATMs) and dendritic cells (ATDCs), are implicated in metabolic inflammation, insulin resistance, and altered regulation of adipocyte function. We evaluated stromal cell and white adipose tissue (WAT) expansion dynamics with high fat diet (HFD) feeding for 3-56 days, quantifying ATMs, ATDCs, endothelial cells (ECs), and preadipocytes (PAs) in visceral epididymal WAT and subcutaneous inguinal WAT. To better understand mechanisms of the early response to obesity, we evaluated ATM proliferation and lipid accumulation. ATMs, ATDCs, and ECs increased with rapid WAT expansion, with ATMs derived primarily from a CCR2-independent resident population. WAT expansion stimulated proliferation in resident ATMs and ECs, but not CD11c+ ATMs or ATDCs. ATM proliferation was unperturbed in Csf2- and Rag1-deficient mice with WAT expansion. Additionally, ATM apoptosis decreased with WAT expansion, and proliferation and apoptosis reverted to baseline with weight loss. Adipocytes reached maximal hypertrophy at 28 days of HFD, coinciding with a plateau in resident ATM accumulation and the appearance of lipid-laden CD11c+ ATMs in visceral epididymal WAT. ATM increases were proportional to tissue expansion and adipocyte hypertrophy, supporting adipocyte-mediated regulation of resident ATMs. The appearance of lipid-laden CD11c+ ATMs at peak adipocyte size supports a role in responding to ectopic lipid accumulation within adipose tissue. In contrast, ATDCs increase independently of proliferation and may be derived from circulating precursors. These changes precede and establish the setting in which large-scale adipose tissue infiltration of CD11c+ ATMs, inflammation, and adipose tissue dysfunction contributes to insulin resistance.
Keywords: adipocyte; adipose tissue dendritic cell; apoptosis; foam cell; obesity.
©2018 Society for Leukocyte Biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Adipose tissue's rapid response team.J Leukoc Biol. 2018 Apr;103(4):611-613. doi: 10.1002/JLB.1CE0118-006R. Epub 2018 Mar 13. J Leukoc Biol. 2018. PMID: 29534319 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

