Dynamic changes in plasma Epstein-Barr virus DNA load during treatment have prognostic value in nasopharyngeal carcinoma: a retrospective study
- PMID: 29493874
- PMCID: PMC5911595
- DOI: 10.1002/cam4.1381
Dynamic changes in plasma Epstein-Barr virus DNA load during treatment have prognostic value in nasopharyngeal carcinoma: a retrospective study
Abstract
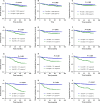
Circulating plasma Epstein-Barr virus DNA (EBV DNA) is related to tumor recurrence and metastasis and has potential as a dynamic, sensitive, and specific marker in nasopharyngeal carcinoma (NPC). We investigated the clinical significance of assessing plasma EBV DNA load at various time points during treatment. Patients with NPC (n = 949) for whom plasma EBV DNA load was measured by real-time quantitative polymerase chain reaction (RT-qPCR) before treatment (pre-EBV) and at midtreatment (mid-EBV), end of treatment (end-EBV), and 3 months after completing treatment (3 m-EBV) were retrospectively assessed. Receiver operating characteristic (ROC) curve analysis was used to identify the optimal EBV DNA cutoff point for each time point. Overall survival (OS), distant metastasis-free survival (DMFS), and progression-free survival (PFS) were compared using Kaplan-Meier estimates. High pre-EBV, high mid-EBV, high end-EBV, and high 3 m-EBV were all associated with significantly poorer OS, DMFS, and PFS in the entire cohort. Detectable end-EBV and 3 m-EBV was associated with significantly poorer OS, DMFS, and PFS. Among patients with detectable end-EBV, adjuvant therapy significantly improved OS (HR 2.419; 95% CI 1.297-4.51, P = 0.03) and DMFS (HR 2.45; 95% CI 1.243-4.828, P = 0.04), but not PFS (P = 0.17). EBV DNA represents a dynamic biomarker for monitoring treatment and predicting survival in NPC. Assessing plasma EBV DNA before, during, and after chemoradiotherapy could be clinically valuable and enable selection of patients most likely to benefit from additional therapy and improve assessment of treatment response and disease surveillance. Further multicenter prospective investigations are warranted.
Keywords: Epstein-Barr virus DNA; nasopharyngeal carcinoma; survival; treatment.
© 2018 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures



References
-
- Lo, Y. M. 2001. Quantitative analysis of Epstein‐Barr virus DNA in plasma and serum: applications to tumor detection and monitoring. Ann. N. Y. Acad. Sci. 945:68–72. - PubMed
-
- Leung, S. F. , Zee B., Ma B. B., Hui E. P., Mo F., Lai M., et al. 2006. Plasma Epstein‐Barr viral deoxyribonucleic acid quantitation complements tumor‐node‐metastasis staging prognostication in nasopharyngeal carcinoma. J. Clin. Oncol. 24:5414–5418. - PubMed
-
- Chen, Q. Y. , Guo S. Y., Tang L. Q., Lu T. Y., Chen B. L., Zhong Q. Y., et al. 2017. Combination of tumor volume and Epstein‐Barr Virus DNA improved prognostic stratification of stage II nasopharyngeal carcinoma in the IMRT Era: a large‐scale cohort study. Cancer research and treatment : official journal of Korean Cancer Association doi: 10.4143/crt.2017.237. - DOI - PMC - PubMed
-
- Lam, J. W. , Chan J. Y., Ho W. K., and Tsang R. K.. 2016. Use of transoral nasopharyngeal brush biopsy for Epstein‐Barr virus DNA detection of local recurrence of nasopharyngeal carcinoma after radiotherapy. Head Neck 38(Suppl 1):E1301–E1304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

