Biliverdin reductase and bilirubin in hepatic disease
- PMID: 29494209
- PMCID: PMC6032063
- DOI: 10.1152/ajpgi.00026.2018
Biliverdin reductase and bilirubin in hepatic disease
Abstract
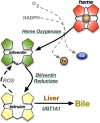
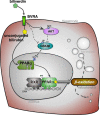
The buildup of fat in the liver (hepatic steatosis) is the first step in a series of incidents that may drive hepatic disease. Obesity is the leading cause of nonalcoholic fatty liver disease (NAFLD), in which hepatic steatosis progresses to liver disease. Chronic alcohol exposure also induces fat accumulation in the liver and shares numerous similarities to obesity-induced NAFLD. Regardless of whether hepatic steatosis is due to obesity or long-term alcohol use, it still may lead to hepatic fibrosis, cirrhosis, or possibly hepatocellular carcinoma. The antioxidant bilirubin and the enzyme that generates it, biliverdin reductase A (BVRA), are components of the heme catabolic pathway that have been shown to reduce hepatic steatosis. This review discusses the roles for bilirubin and BVRA in the prevention of steatosis, their functions in the later stages of liver disease, and their potential therapeutic application.
Keywords: ALD; BVRA; NAFLD; PPARα; bilirubin; fatty liver disease; fibrosis.
Figures
References
-
- Abbasi A, Deetman PE, Corpeleijn E, Gansevoort RT, Gans RO, Hillege HL, van der Harst P, Stolk RP, Navis G, Alizadeh BZ, Bakker SJ. Bilirubin as a potential causal factor in type 2 diabetes risk: a Mendelian randomization study. Diabetes 64: 1459–1469, 2015. doi:10.2337/db14-0228. - DOI - PMC - PubMed
-
- Belo L, Nascimento H, Kohlova M, Bronze-da-Rocha E, Fernandes J, Costa E, Catarino C, Aires L, Mansilha HF, Rocha-Pereira P, Quintanilha A, Rêgo C, Santos-Silva A. Body fat percentage is a major determinant of total bilirubin independently of UGT1A1*28 polymorphism in young obese. PLoS One 9: e98467, 2014. doi:10.1371/journal.pone.0098467. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

