Automated high throughput microscale antibody purification workflows for accelerating antibody discovery
- PMID: 29494273
- PMCID: PMC5973699
- DOI: 10.1080/19420862.2018.1445450
Automated high throughput microscale antibody purification workflows for accelerating antibody discovery
Abstract
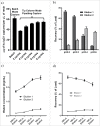
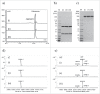
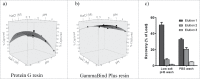
To rapidly find "best-in-class" antibody therapeutics, it has become essential to develop high throughput (HTP) processes that allow rapid assessment of antibodies for functional and molecular properties. Consequently, it is critical to have access to sufficient amounts of high quality antibody, to carry out accurate and quantitative characterization. We have developed automated workflows using liquid handling systems to conduct affinity-based purification either in batch or tip column mode. Here, we demonstrate the capability to purify >2000 antibodies per day from microscale (1 mL) cultures. Our optimized, automated process for human IgG1 purification using MabSelect SuRe resin achieves ∼70% recovery over a wide range of antibody loads, up to 500 µg. This HTP process works well for hybridoma-derived antibodies that can be purified by MabSelect SuRe resin. For rat IgG2a, which is often encountered in hybridoma cultures and is challenging to purify via an HTP process, we established automated purification with GammaBind Plus resin. Using these HTP purification processes, we can efficiently recover sufficient amounts of antibodies from mammalian transient or hybridoma cultures with quality comparable to conventional column purification.
Keywords: antibody; automation; high throughput; hybridoma; liquid handling system; purification; rat IgG2a; small scale; tip column; transient expression.
Figures



References
-
- Grodzki AC, Berenstein E. Antibody purification: Affinity Chromatography – Protein A and Protein G Sepharose In Oliver C, Jamur MC, eds. Immunocytochemical Methods and Protocols. Totowa, NJ: Humana Press, 2010:33–41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
