Comparison of Growth of Healthy Term Infants Fed Extensively Hydrolyzed Protein- and Amino Acid-Based Infant Formulas
- PMID: 29494498
- PMCID: PMC5872707
- DOI: 10.3390/nu10030289
Comparison of Growth of Healthy Term Infants Fed Extensively Hydrolyzed Protein- and Amino Acid-Based Infant Formulas
Abstract
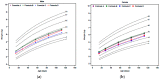
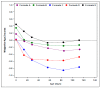
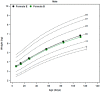
The aim of this narrative review was to assess published growth data for healthy, term, infants consuming extensively hydrolyzed protein-based (EHF), or amino acid-based formulas (AAF). These data may be of use to clinicians managing infants with medical conditions consuming these products. A search was conducted using key terms: amino acid-based, hydrolysate, hydrolyzed, hydrolysed, infant formula, infant formulae or formulas, baby formula, or formulae or formulas, infant, infants, infantile, and growth. Seven controlled, randomized, prospective growth trials of healthy term infants fed EHFs or AAFs at similar time points during the first four months of age met these and other criteria, including that the trial was published in a peer-reviewed journal, subjects were enrolled by ≤14 days of age and were exclusively formula-fed at entry and throughout the duration of the trial, and infants were assessed at regular intervals with weight measures available ideally at 14 days, one, two, three, and four months of age. Results suggested that healthy infants receiving commonly available EHFs and AAFs do not appear to experience accelerated growth as reported for infants fed many standard formulas. Differences in growth patterns were observed with some formulas supporting normative growth patterns during the first four months but others appearing to support markedly lower growth patterns. These observations should be confirmed in well-designed prospective randomized trials. Until that time, it is recommended that EHFs and AAFs be chosen carefully with individual patient needs considered.
Keywords: elemental formula; extensively hydrolyzed protein-based formula; free amino acid-based formula; hydrolysate formula; infant growth.
Conflict of interest statement
M.W.B., G.E.B. and J.S.O. are employees of Abbott Nutrition, Abbott Laboratories, the manufacturer of two of the formulas included in this review.
Figures
Comment in
-
Letter to the Editor Re: Borschel M., et al. Comparison of Growth of Healthy Term Infants Fed Extensively Hydrolyzed Protein- and Amino Acid-Based Infant Formulas. Nutrients 2018, 10, 289.Nutrients. 2019 Jan 17;11(1):185. doi: 10.3390/nu11010185. Nutrients. 2019. PMID: 30658383 Free PMC article.
References
-
- Dewey K.G., Peerson J.M., Brown K.H., Krebs N.F., Michaelsen K.F., Persson L.A., Salmenpera L., Whitehead R.G., Yeung D.L., World Health Organization Working Group on Infant Growth Growth of breast-fed infants deviates from current reference data: A pooled analysis of US, Canadian, and European data sets. Pediatrics. 1995;96:495–503. - PubMed
-
- World Health Organization . The Growth Chart: A Tool for Use in Infant and Child Health Case. World Health Organization; Geneva, Switzerland: 1986.
-
- Hamill P.V.V., Drizd T.A., Johnson C.L., Reed R.B., Roche A.F. NCHS Growth Curves for Children, Birth-18 Years. Department of Health, Education, and Welfare, Public Health Service, [Health Resources Administration], National Center for Health Statistics; Washington, DC, USA: 1977. - PubMed
-
- World Health Organization Multicentre Growth Reference Study Group . WHO Child Growth Standards: Length/Height-For-Age, Weight-For-Age, Weight-For-Length, Weight-For-Height and Body Mass Index-For-Age: Methods and Development. World Health Organization; Geneva, Switzerland: 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

