Trimethylamine-N-Oxide (TMAO)-Induced Impairment of Cardiomyocyte Function and the Protective Role of Urolithin B-Glucuronide
- PMID: 29494535
- PMCID: PMC6017162
- DOI: 10.3390/molecules23030549
Trimethylamine-N-Oxide (TMAO)-Induced Impairment of Cardiomyocyte Function and the Protective Role of Urolithin B-Glucuronide
Abstract
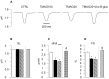
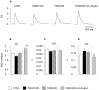
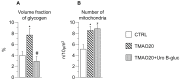
One of the most recently proposed candidates as a potential trigger for cardiovascular diseases is trimethylamine-N-oxide (TMAO). Possible direct effects of TMAO on myocardial tissue, independent of vascular damage, have been only partially explored so far. In the present study, we assessed the detrimental direct effects of TMAO on cardiomyocyte contractility and intracellular calcium dynamics, and the ability of urolithin B-glucuronide (Uro B-gluc) in counteracting TMAO-induced cell damage. Cell mechanics and calcium transients were measured, and ultrastructural analysis was performed in ventricular cardiomyocytes isolated from the heart of normal adult rats. Cells were either untreated, exposed to TMAO, or to TMAO and Uro B-gluc. TMAO exposure worsened cardiomyocyte mechanics and intracellular calcium handling, as documented by the decrease in the fraction of shortening (FS) and the maximal rate of shortening and re-lengthening, associated with reduced efficiency in the intracellular calcium removal. Ultrastructurally, TMAO-treated cardiomyocytes also exhibited glycogen accumulation, a higher number of mitochondria and lipofuscin-like pigment deposition, suggesting an altered cellular energetic metabolism and a higher rate of protein oxidative damage, respectively. Uro B-gluc led to a complete recovery of cellular contractility and calcium dynamics, and morphologically to a reduced glycogen accumulation. We demonstrated for the first time a direct negative role of TMAO on cardiomyocyte functional properties and the ability of Uro B-gluc in counteracting these detrimental effects.
Keywords: Trimethylamine-N-oxide; cardiomyocyte mechanics; cardiovascular diseases; ellagitannins; urolithins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- World Health Organization (WHO) Global Status Report on Noncommunicable Diseases 2014. WHO; Geneva, Switzerland: 2014.
-
- Li X.S., Obeid S., Klingenberg R., Gencer B., Mach F., Raber L., Windecker S., Rodondi N., Nanchen D., Muller O., et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017;38:814–824. doi: 10.1093/eurheartj/ehw582. - DOI - PMC - PubMed
-
- Meyer K.A., Benton T.Z., Bennett B.J., Jacobs D.R., Jr., Lloyd-Jones D.M., Gross M.D., Carr J.J., Gordon-Larsen P., Zeisel S.H. Microbiota-Dependent Metabolite Trimethylamine N-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA) J. Am. Heart Assoc. 2016;5:e003970. doi: 10.1161/JAHA.116.003970. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

