Translational Advances of Hydrofection by Hydrodynamic Injection
- PMID: 29494564
- PMCID: PMC5867857
- DOI: 10.3390/genes9030136
Translational Advances of Hydrofection by Hydrodynamic Injection
Abstract
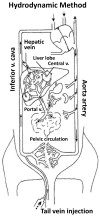
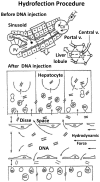
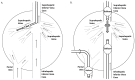
Hydrodynamic gene delivery has proven to be a safe and efficient procedure for gene transfer, able to mediate, in murine model, therapeutic levels of proteins encoded by the transfected gene. In different disease models and targeting distinct organs, it has been demonstrated to revert the pathologic symptoms and signs. The therapeutic potential of hydrofection led different groups to work on the clinical translation of the procedure. In order to prevent the hemodynamic side effects derived from the rapid injection of a large volume, the conditions had to be moderated to make them compatible with its use in mid-size animal models such as rat, hamster and rabbit and large animals as dog, pig and primates. Despite the different approaches performed to adapt the conditions of gene delivery, the results obtained in any of these mid-size and large animals have been poorer than those obtained in murine model. Among these different strategies to reduce the volume employed, the most effective one has been to exclude the vasculature of the target organ and inject the solution directly. This procedure has permitted, by catheterization and surgical procedures in large animals, achieving protein expression levels in tissue close to those achieved in gold standard models. These promising results and the possibility of employing these strategies to transfer gene constructs able to edit genes, such as CRISPR, have renewed the clinical interest of this procedure of gene transfer. In order to translate the hydrodynamic gene delivery to human use, it is demanding the standardization of the procedure conditions and the molecular parameters of evaluation in order to be able to compare the results and establish a homogeneous manner of expressing the data obtained, as 'classic' drugs.
Keywords: gene therapy; hydrodynamic; non-viral; translational.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Crepso J. Long-term expression of the human alpha1-antitrypsin gene in mice employing anionic and cationic liposome vectors. Biochem. Pharmacol. 1996;51:1309–1314. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

